
Background
Hybridization has been shown to play a critical role in the evolution of plants (Rieseberg and Wendel 1993) and molecular studies have allowed scientists to re-evaluate taxonomy through multiple methods besides just morphological characteristics (Li et al. 2012). However, some ferns, such as the cheilanthoid ferns in the family Pteridaceae, had not been re-classified as of 2012 even though they were known to be non-monophyletic (that they didn’t all share a single common ancestor). Li et al. (2012) were particularly interested in a group of about twenty cheilanthoid ferns referred to as the “Cheilanthes marginata group,” which are found in arid habitats ranging from Arizona and Texas all the way to Bolivia. They hoped to distinguish this group from its most closely related fern relatives and revise the taxonomy based on the number of chromosomes (an organism’s condensed DNA) and genetic relatedness.
To be able to study and appreciate the fern family tree a little background is first needed on the fern life cycle, which varies from flowering plants. It can generally be described in two distinct stages: the sporophyte, which is diploid (has the full set of chromosomes) and is what we recognize as the adult fern, and the gametophyte, which is haploid (has half the number of chromosomes as the sporophyte) (Judd et al. 1999, Moran 2004) (see Fig. 1 for a gametophyte). Ferns don’t produce seeds and instead have spores on the underside of their leaves, which are released and germinate and become gametophytes (Judd et al. 1999, Moran 2004). The gametophyte has both male and female sex organs, which produce the appropriate sperm and eggs and usually require water to help the sperm travel to the eggs for fertilization (Judd et al. 1999, Moran 2004). A sporophyte, with a full set of chromosomes as well as leaves and a root, then sprouts from the fertilized gametophyte and grows to become a fully developed adult fern, beginning the cycle again (Fig. 2) (Judd et al. 1999, Moran 2004).
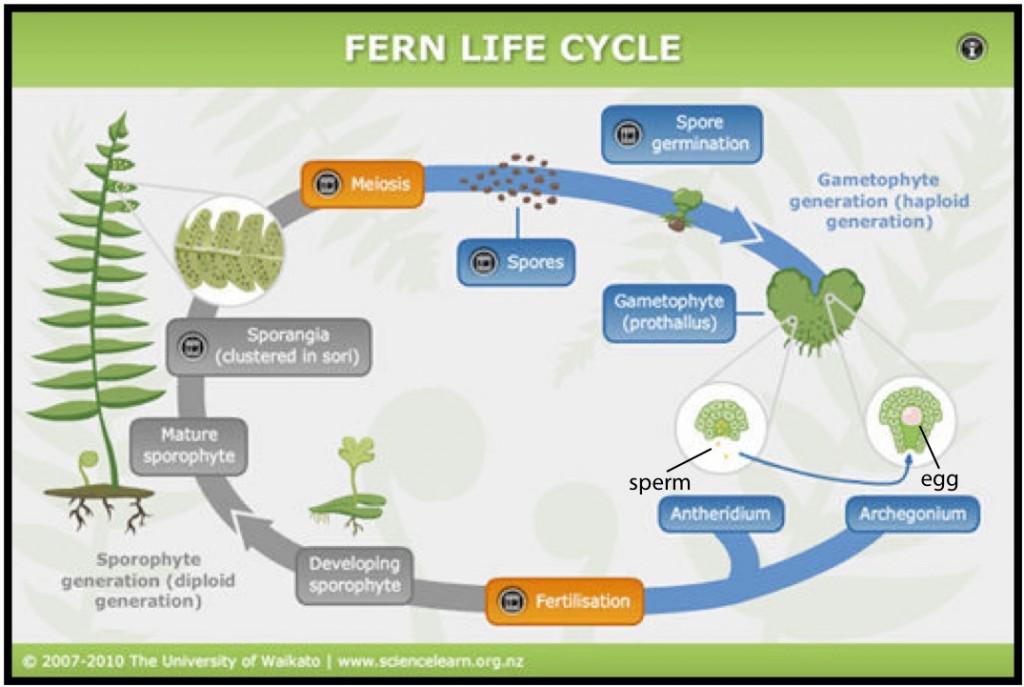
Approximately 5-10% of all fern species are asexual and produce a sporophyte from the gametophyte without fertilization (Moran 2004, Sheffield 2008). This is more common in species that live in arid environments, like the “C. marginata group,” perhaps because the lack of water gives them an advantage (Moran 2004, Sheffield 2008); they just create a clone of themselves! The majority of asexually reproducing ferns also tend to have more than two sets of chromosomes – these are called polyploids (Moran 2004).
Evidence
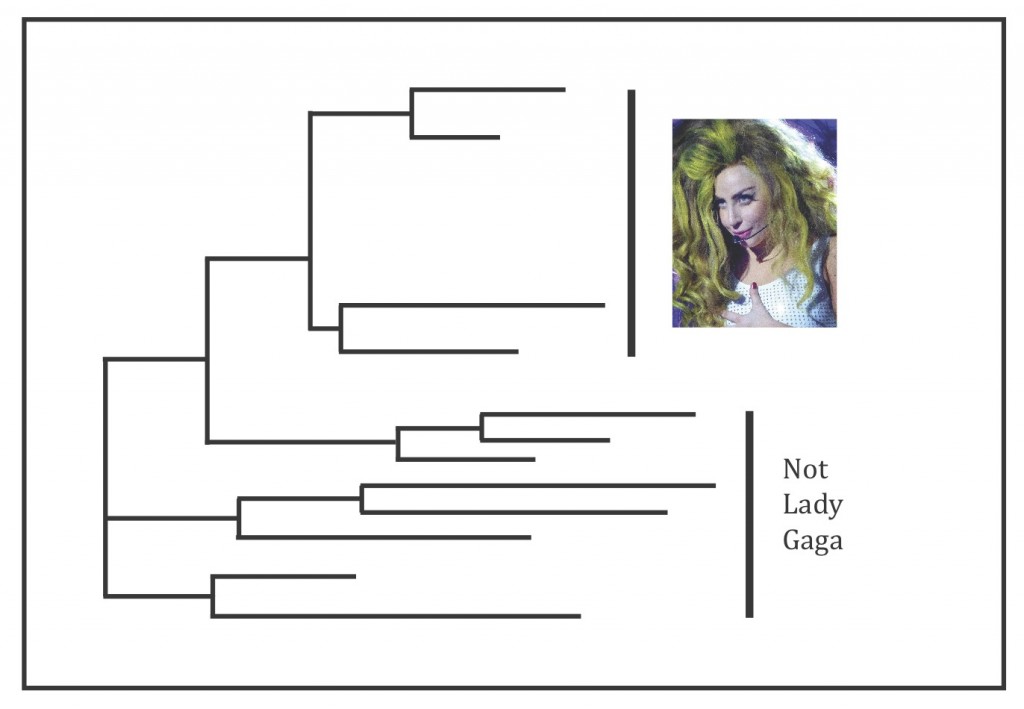
In order to tease apart the complex relationships within the “C. marginata group,” Li et al. had to determine the number of chromosomes each fern has (ploidy level), how they reproduce, as well as how they’re all related to each other through molecular analyses. There were a lot of results but the punch line is Li et al. think the “C. marginata group” deserves to be its own genus, which they’ve named Gaga Pryer, Fay-Wei Li, & Windham, containing nineteen species. Two of these species are brand new: Gaga germanotta Fay-Wei Li & Windham and G. monstraparva Fay-Wei Li & Windham, both of which are direct nods to Lady Gaga (Fig. 1) (Li et al. 2012).
Li et al. generally found that the sexually reproducing species had haploid spores, meaning they only have half of the total number of required chromosomes (Moran 2004). The other samples that Li et al. found reproduce asexually all had polyploid spores (more than two sets of chromosomes). The resulting phylogenetic trees from the molecular analyses all showed the “C. marginata group” forming a monophyletic clade (Fig. 3). The sample representing the type species of the genus Cheilanthes, the original genus that the “C. marginata group” was described under, did not fall within this monophyletic clade and was not shown to be the most closely related fern to it either – further evidence that the “C. marginata group” didn’t belong in the genus Cheilanthes (Li et al. 2012).
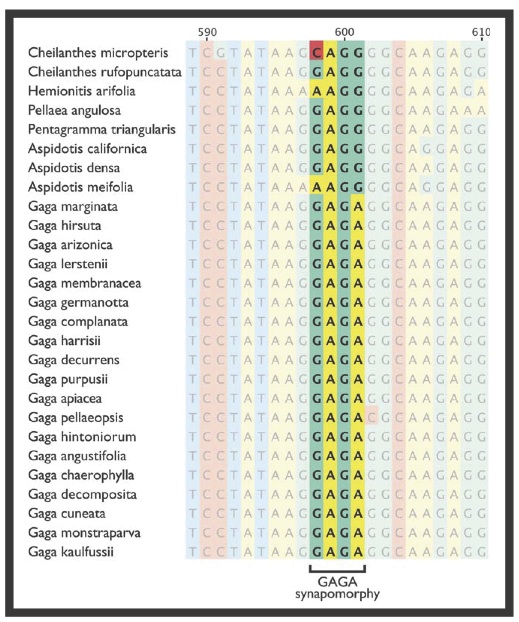
While analyzing the genetic sequences used to produce phylogenetic trees, Li et al. noticed a four base pair long difference that the “C. marginata group” had from the rest of the samples – a mutation that read GAGA instead of CAGG or GAGG (Fig. 4). This molecular data, in conjunction with the morphological differences between the “C. marginata group” and its most closely related fern relatives, are strong evidence in support of the authors’ decision to separate the “C. marginata group” into its own genus. Within this newly named Gaga genus, Li et al. determined which of the species are hybrids and who some of the parents are by combining their new knowledge of each samples’ ploidy level, method of reproducing, and the molecular data. Four of the Gaga species have been identified as hybrids, including the two brand new species with a possible fifth species that requires more data. They also weren’t able to identify both parents of all of the hybrids, and hope to determine these in future studies (Li et al. 2012).
Follow-up questions
Hybrid ferns often have multiple origins in different areas that the parental plants come into contact with each other (Moran 2004, Werth et al. 1985) and I’m curious whether the Gaga hybrids have occurred multiple times as well or just once. Werth et al. (1985) were able to detect multiple origins of hybrid spleenworts (Asplenium sp.), another genus of ferns, by looking for variations in nine genetic loci shared by their six study species. This could be feasible with the Gaga ferns as well, given more time and money to complete the necessary DNA sequencing. It would also be interesting to know whether any of these hybrids are able to successfully crossbreed with either of their parent species. This could easily be attempted in a lab setting, which would show whether it’s possible, but not necessarily how likely it is to occur in nature.
Some might ask why knowing how these ferns are related to each other is important or just wonder in general why we should care about taxonomy is. But knowing what species exist in the world is vital for trying to understand biodiversity, and necessary for conservation (Mace 2004, Schlick-Steiner et al. 2010). After all, we can’t adequately protect species if we can’t identify what they are and with only about 1.7 million species described out of the estimated 7-15 million species in total we certainly have a long way to go (Mace 2004). But Li et al. are doing their part, one fern genus at a time.
Further reading
Natural History of Ferns by Robbin C. Moran – I found this to be an interesting book and written in such a way that it’s engaging even for non-fern folks. It also has some beautifully drawn figures for those who love botanical illustrations!
Science Learning has some nice resources on ferns for teachers and younger students, as well as an abundance of other science topics covering many disciplines. Most of the research discussed on the site pertains to New Zealand but it also has useful videos and interactive tools on general fern physiology and life history.
The Pryer Lab at Duke University has published many other interesting fern papers including a more recent one on a fern hybrid whose parents speciated from each other about 60 million years ago; it’s the most deeply diverged hybridization event currently known (Rothfels et al. 2015).
References
Judd, W.S., Campbell, C.S., Kellogg, E.A., and Stevens, P.F. 1999. Plant Systematics: A Phylogenetic Approach. Sinauer Associates, Inc., Sunderland, Mass.
Li, F.W., Pryer, K.M., and Windham, M.D. 2012. Gaga, a new fern genus segregated from Chelianthes (Pteridaceae). Systematic Botany 37(4): 845-860. doi:10.1600/036364412X656626.
Mace, G.M. 2004. The role of taxonomy in species conservation. Philosophical Transactions B. 359: 711-719. doi:10.1098/rstb.2003.1454.
Moran, R.C. 2004. A Natural History of Ferns. Timber Press, Inc., Portland, Ore.
Rieseberg, L.H. and Wendel, J.F. 1993. Introgression and its consequences in plants. In Hybrid Zones and the Evolutionary Process. Edited by R.G. Harrison. Oxford University Press, Inc.. New York. pp. 70-110.
Rothfels, C.J., Johnson, A.K., Hovenkam, P.H., Swofford, D.L., Roskam, H.C., Fraser-Jenkins, C.R., Windham, M.D., and Pryer, K.M. 2015. Natural hybridization between genera that diverged from each other approximately 60 million years ago. American Naturalist 185: 433-442. doi:10.1086/679662.
Schlick-Steiner, B.C., Steiner, F.M., Seifert, B., Stauffer, C., Christian, E., and Crozier, R.H. 2010. Integrative taxonomy: a multisource approach to exploring biodiversity. Annual Review of Entomology. 55: 421-438. doi:10.1146/annurev-ento-112408-085432.
Sheffield, E. 2008. Alternation of generations. In Biology and Evolution of Ferns and Lycophytes. Edited by T.A. Ranker and C.H. Haufler. Cambridge University Press. Cambridge, U.K.. pp. 49-74.
Werth, C.R., Guttman, S.I., and Eshbaugh, W.H. 1985. Recurring origins of allopolyploid species in Asplenium. Science. 228(4700): 731-733. https://www.jstor.org/stable/1694549.
