Background:
Over the pasting years, atherosclerosis continues to be the number one killer in the United States (Heart disease facts 2021). This chronic inflammatory disorder is the foundation of heart disease (CVD) and happens when dietary cholesterol builds up in our arteries. With the accumulation of cholesterol comes arterial wall damage and the prevention of oxygen-rich blood circulating smoothly throughout the body. Fatty streaks then progress into advanced lesions followed by strokes, heart attacks, and death.
There have been several suggestions to decrease the illness and mortality of CVD. Statins have been prescribed to patients to reduce the production of cholesterol. Alternatively, stents have been surgically inserted in patients to keep blood flowing through narrowed arteries. Yet, the mortality rate from CVD continues to rise. Researchers propose to tackle this issue at a different angle: the gut microbiome. Does your gut health play a role in promoting CVD? You bet it does. Previous studies have suggested that the excessive consumption of animal products along with their interactions with the gut microbiome can play a substantial role in the progression of CVD (Witkowski et al., 2020).
So who is the new player of interest in the scientific community in regards to CVD? The novel player is trimethylamine oxide (TMAO) (Goldsmith & Sartor, 2014). TMAO is a molecule that comes from our gut microbes when it ingests dead animal bacteria from dietary meat. This harmful molecule then gets processed by the liver and may aid in attaching the cholesterol onto our arterial walls (Koeth et al., 2013). In Figure 1, we’re able to visualize how dietary meat interacts with the gut microbiome, converting to TMAO, and its contribution to chronic diseases.
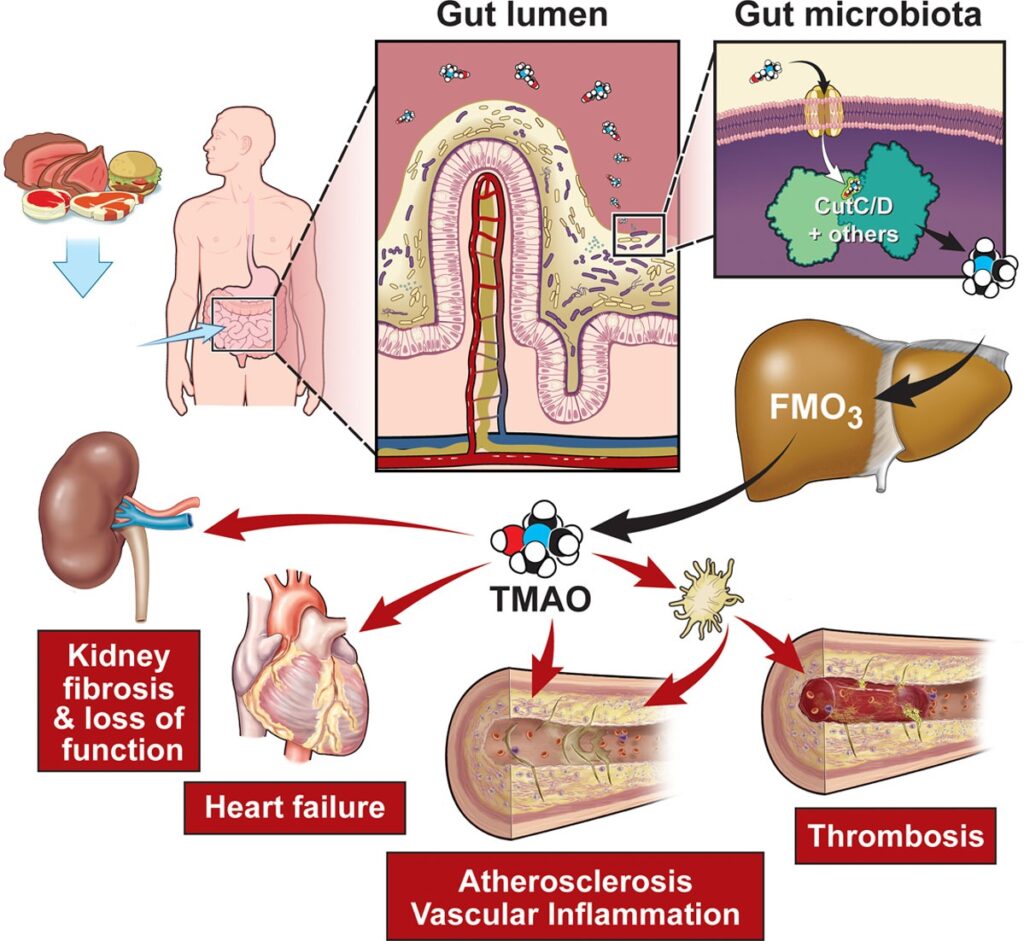
Researchers have yet to indicate which specific gut microbes trigger the production of TMAO, but some are evaluating TMAO levels in our bloodstream to indicate the risk of CVD. A study conducted by the American Heart Journal had followed a population size of 2,181 for 8 years and found that the cutoff level of TMAO in a plasma blood sample for CVD risk was at 1.5uM- 10.5uM (Tang et al., 2021). Numerous studies have also indicated TMAO levels in plasma blood samples, yet, the finite amount of TMAO levels that indicate the risk of CVD remains unclear.
The Underlying Question:
Would an Oral Carnitine Challenge Test (OCCT) be a better marker for identifying high TMAO levels in patients? If so, what kind of phenotypes are within the human gut microbiome that would increase the production of TMAO?
Evidence:
Before officially using the OCCT to analyze TMAO levels in patients, Wu et al., (2018) recruted a study population and subjected them to a Food Frequency Questionnaire (FFQ) dietary assessment. This questionnaire measures how often someone eats different foods. One question can ask how many times you put cream in your coffee. Another question can ask how many servings of vegetables do you have in one day. Based on the FFQ responses, the participants were categorized as either an omnivore or a vegetarian. With this information, the authors wanted to clarify whether there is a difference in the gut microbiome of these two diets. What they found was that although participants had different diets, all participants shared similar gut bacteria. This finding is important for the authors’ experiment because since TMAO comes from the gut, any differences seen with TMAO production will solely be based on the participants’ diet.
The next phase for this preliminary study was to test the effectiveness of a carnitine supplement. What is carnitine and how does carnitine tie into the story? Carnitine is essentially the compound that comes from the dead animal bacteria from dietary meat that aids in the production of TMAO. Once the participants ingested the carnitine, they were then subjected to six blood tests. Due to the amount of TMAO in each sample, the authors deduced that evaluating TMAO production was effective at 24 hours and 48 hours.
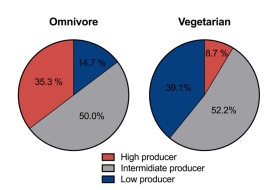
After the participants’ diets were assessed and effective timepoints for evaluating TMAO production were established, the oral carnitine challenge test (OCCT) took place. This consisted of the 57 participants (23 vegetarians and 34 omnivores) fasting and ingesting carnitine tablets. The participants were then subjected to blood and urine sampling at 24 and 48 hours for TMAO abundance. After measuring the levels of TMAO within the samples, the authors’ found that omnivores and vegetarians had different abilities to transform the carnitine supplements into TMAO. In Figure 2, the color red represents high TMAO production, grey indicates intermediate TMAO production and blue defines low TMAO production. It is observed that omnivores were more likely than vegetarians to produce higher amounts of TMAO. For this reason, the authors’ suggested that the OCCT is sufficient in identifying high TMAO levels in patients (Wu et al., 2018).
After establishing the quality of OCCT in regards to TMAO production, the gut microbiome between high TMAO producers and low TMAO producers was investigated. Fecal samples from the subjects were taken and then analyzed on a microbiome database. These researchers found that there was a similarity between the gut microbiome composition of high TMAO producers and low TMAO producers. In addition, participants that produced high amounts of TMAO exhibited a decreased abundance of a bacteria called Bacteroidetes. This indicates that these subjects had lower amounts of bacteria that break down plant fiber and convert it to energy. In order to translate the authors’ findings, fecal samples from the high TMAO participants and low TMAO participants were transplanted to germ-free mice. The production of TMAO was significantly reproduced in the mice, thus suggesting that certain phenotypes within the gut microbiome can increase the ability to produce TMAO.
What’s Next?:
The OCCT method developed by Wu et al., (2018) can be used to determine the production of TMAO by evaluating the gut microbiota of omnivores and vegetarians. However, the human gut microbiome is more unique to a single individual. In turn, I propose that the next step could be an extensive study that reproduces the authors’ findings but on a broader scope. These future studies will consist of reproducing the authors’ work using a comprehensive study population ranging from various ethnicities and ages. Based on various gut microbiomes from other ethnicities’ diets, we’ll be able to see how their gut microbiome interacts with a surplus of TMAO consumption. This will give insight as to whether other ethnicities and their influenced diets can combat TMAO production. A meta-analysis of these studies can then establish the mechanism of TMAO and its production capacity TMAO-producer phenotypes within the human gut microbiome.
Once these studies are evaluated, pharmaceutical companies could produce a drug that either eliminates the gut phenotype involved in TMAO synthesis for human subjects. Pharmaceutical companies could also generate a drug that could degrade dietary carnitine and choline within the animal cell walls. These drugs could be administered to the animal a couple of days before the slaughter. The production of dietary carnitine may be less understood, but if clarified, dietary carnitine and choline could be eliminated from the human diet. Another approach would be using these studies to aid in personalizing nutritional plans for CVD risk patients.
Feed Your Curiosity:
How far has science gotten in understanding our gut health and chronic diseases? Click HERE
What happens to our gut when we start to eat more vegetables? Click HERE
Want to know the inside scoop on TMAO? Click HERE
Are you interested in how doctors are changing their patients’ gut health to cure heart disease? Click HERE
References:
- Centers for Disease Control and Prevention. (2021, September 27). Heart disease facts. Centers for Disease Control and Prevention. Retrieved October 11, 2021, from https://www.cdc.gov/heartdisease/facts.htm.
- Goldsmith, J. R., & Sartor, R. B. (2014). The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. Journal of Gastroenterology, 49(5), 785–798. https://doi.org/10.1007/s00535-014-0953-z
- Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., Britt, E. B., Fu, X., Wu, Y., Li, L., Smith, J. D., DiDonato, J. A., Chen, J., Li, H., Wu, G. D., Lewis, J. D., Warrier, M., Brown, J. M., Krauss, R. M., … Hazen, S. L. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine, 19(5), 576–585. https://doi.org/10.1038/nm.3145
- Tang, W. H. W., Li, X. S., Wu, Y., Wang, Z., Khaw, K.-T., Wareham, N. J., Nieuwdorp, M., Boekholdt, S. M., & Hazen, S. L. (2021). Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the epic-Norfolk prospective population study. American Heart Journal, 236, 80–86. https://doi.org/10.1016/j.ahj.2021.01.020
- Witkowski, M., Weeks, T. L., & Hazen, S. L. (2020). Gut microbiota and cardiovascular disease. Circulation Research, 127(4), 553–570. https://doi.org/10.1161/circresaha.120.316242
- Wu, W.-K., Chen, C.-C., Liu, P.-Y., Panyod, S., Liao, B.-Y., Chen, P.-C., Kao, H.-L., Kuo, H.-C., Kuo, C.-H., Chiu, T. H., Chen, R.-A., Chuang, H.-L., Huang, Y.-T., Zou, H.-B., Hsu, C.-C., Chang, T.-Y., Lin, C.-L., Ho, C.-T., Yu, H.-T., … Wu, M.-S. (2018). Identification of TMAO-producer phenotype and host–diet–gut dysbiosis by Carnitine Challenge Test in human and germ-free mice. Gut, 68(8), 1439–1449. https://doi.org/10.1136/gutjnl-2018-317155
