Background:
Anxiety and depression are at an all-time high, with more diagnoses and higher disability ratings (Spitchak, 2020). Mental health disorders like this have been linked to inflammation in the gut, as the immune system is activated by stress (Spitchak, 2020). Cognitive Behavioral Therapy and anxiety-reducing medications like benzodiazepines are the current gold standard for managing long-term chronic stress and anxiety, as well as short-term needs like panic or anxiety attacks (Ballenger, 2000). These methods do help, but some people have drug-resistant depression, and others simply prefer a greater variety of options to manage their symptoms. Getting outside more has long been accepted as a mental health boost, both from the calming peaceful environment to the vitamin D exposure, to the clean air, and even the soil microbes (Asprey, 2021). A less conventional option than a simple walk may exist and appears to be emerging after 16 plus years of research by Chris Lowry, who has been working with the soil bacterium M. vaccae since the early 2000s.
The biggest question is, can microbes actually help to relieve stress? Some people think so, enough to invest in the bacterial supplement market. A new supplement has been released in a partnership between Immunitor, a company specializing in the oral delivery of vaccines, and Key Capital Corporation, a natural resource investment company. The supplement contains Mycobacterium vaccae with ionic Magnesium to stabilize, with hopes for targeted relief for anxiety and stress. In more concrete terms, science supports this idea as well. Research on a mouse model by Stefan Reber et al (2016), including Chris Lowry, shows findings that support the idea that M. vaccae may play a role in lowering anxiety rates and this pattern may be transferable to humans.
Purpose:
Reber and his colleagues were looking to find evidence that supports or fails to support the hypothesis that an injection of heat-killed M. vaccae could protect mice from future stress-induced submissive behaviors, colitis or worsening colitis, and gut microbiota changes.
Evidence:
Reber et al (2016) shows correlations between immunizations with heat-killed M. vaccae and lowered anxiety-like behaviors in stressed mice. They also found increased health of the gut, shown through colitis rates and severity.
Reber et al (2016) found that immunization with M. vaccae helped mice cope with stress. Stressed and immunized mice had a healthier gut: they produced significantly more anti-inflammatory protein and less inflammatory proteins than the untreated controls. The stressed mice who had been immunized have significantly more IL-10, the antiinflammatory protein, compared to the vaccinated but not stressed mice, and have increased levels compared to all the non immunized mice. This suggests in order to have this high anti-inflammatory property, the mice need to be immunized as well as under stress. The stressed mice who had been immunized also had significantly low levels of IL-6, the pro-inflammatory protein, compared to the stressed mice without the immunization.
This combination of inflammatory proteins worked together to protect the colon tissue and resulted in a significant lack of damage to the colon of immunized stressed mice (dark red bar) compared to stressed mice without the bacterium (dark blue bar). Samples of the colon were taken from the mice, and the tissue was examined and rated by the damage observed. For reference, the stress did cause significant damage to the colon of stressed mice without treatment (dark blue bar), compared to unstressed mice without treatment (light blue bar), so we know that the stress causes colon damage and also that the immunization with the bacterium prevents extensive damage.
Image 2: Legend and graphs from Figure 2A in Reber et al (2016). Colon Pathology Histological Damage Score, significant differences between vehicle immunized mice in nonstress and stress environments, and between stressed mice with vehicle immunization and M. vaccae immunization.
The blue bars show mice who are not immunized, and the red bars show immunized mice. The lighter versions (light blue and pink) show unstressed mice, while the darker bars (navy and red) show stressed mice.
In a second test, mice who had damaged colons caused by chemicals, but who were protected by the bacterium, did not worsen in condition under stress. The bacterium also helped stabilize gut microbiome diversity and maintained effectiveness by protecting against colitis despite any changes that did occur. The researchers tested this by taking samples of the mouse feces at a set interval every few days and measured alpha diversity, or the measure of variance within a single mouse’s microbiome over time, as well as beta diversity, or the variance in microbiomes between the stressed and unstressed mouse groups.
The mice with the immunization had higher alpha diversity over time: they kept their microbiomes more stable than their unimmunized counterparts, and also had lower beta diversity, or smaller variance between the stressed mice and the unstressed mice, compared to the two groups of mice without the vaccine. In some taxa of bacteria, such as Helicobacter, Paraprevotella, and Helicobacteraceae, stress increased their number for both immunized and unimmunized mice, and Mucispirrilum decreased. The immunization with M. vaccae did help stabilize these effects, and also resulted in less colon damage despite changes that did occur that give rise to the potential for colitis.
In addition, mice who were treated with the bacterium were more confident, and less submissive than mice without the treatment, as shown by both their behavior in a stressful environment, as well as in maze platform tests. The maze test looks at the time the mice spend on a glass platform that lacks a fence. The mice who spend a long time on this precarious ledge are thought to be more confident and less afraid.
However, the effects of this bacterium depend on the immune response of the mice too: an immune cell called Treg affects how protected the mice are. Mice who had been immunized were further treated with a protein that reduces the amount of Treg, and the gold bar in Image 3 shows that the bacterium only helps reduce the effects of stress on the colon if the immune system of the mouse is working properly. Anxious behavior in a stressful situation is not affected by lowered levels of Treg, but anxious behavior in the maze test is increased. This shows that mice need both the soil bacterium as well as a healthy immune system to prevent stress-induced behaviors and physical damage.
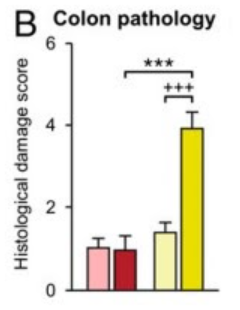
Image 3: Legend and graphs from Figure 3B Reber et al (2016). All the mice are immunized with M. vaccae. Colon pathology, significant differences between stressed mice with unchanged versus minimal treg, and between mice with minimal Treg that were both stressed and unstressed.
All mice shown here are immunized with M. vaccae. Pink and red bars show mice without immune modification (with Treg), and the yellow bars show mice with minimal Treg. Darker versions (red and gold) show stressed mice while lighter bars (pink and yellow) are unstressed.
The soil bacterium also changes the brain of mice. Tph2 and slc6a4 are genes affected by the immunizing bacterium, regardless of stress levels. Tph2 is a gene that gives rise to the production of serotonin, the happiness hormone. In vaccinated mice, there is more gene expression of this gene, suggesting anti-depressive effects in the brain, as measured by mRNA amount. slc6a4 is a gene that regulates the recycling of serotonin, as in stopping the happiness effects of the neurotransmitter and reducing the emotion a happy event triggers. This gene was also measured in expression by mRNA levels and was found to be decreased in expression by the immunization. This suggests not only does M. vaccae increase serotonin to lift mood but also increases the time the hormone can interact in the brain and increase happiness, sort of like a traditional SSRI, a type of depression medication. These genes are implicated in stress resilience and immunization helped lead to calmer, more confident mice. The bacterium also helped increase brain mass in the fear and anxiety parts of the brain, as measured by the mass of the microglia, or specialized brain cells, in target areas of the brain in the prefrontal cortex. Overall, the effects of this bacterium lasted for weeks in the mice, suggesting that this bacterium may have a regular, infrequent use in humans, rather than a daily pill or shot.
Looking Forward:
This bacterium has a promising future and more studies can help confirm the way it affects mice and even humans. Some of the most pressing issues before the bacterium can be reliably used in humans include how the bacterium should be delivered. In Reber et al.’s experiment, they used a heat-killed injection. In the supplement released earlier this year, it’s an oral pill. How does this affect uptake and effects of the bacterium? Humans are a lot bigger than mice and may process the bacterium faster especially depending on the mode of delivery. What does the half-life look like for the bacterium’s effects in humans, and what quantities are needed for dosing? Are there any side effects in humans that behavior and tissue studies on mice can’t tell us? This bacterium needs a lot of human testing before solid results can be claimed, but maybe one day a more organic approach to mental health can be FDA-approved: M. vaccae as a treatment for anxiety.
Further reading:
Chris Lowry’s lab has a central hub located here on research gate. Various papers by him and his peers on this subject and related subjects are located here, as well as information about Lowry’s position at the University of Colorado Boulder and his education and lab goals.
Lighter reading includes these articles by Travel and Leisure and Enjoy Second Summer, which summarize the findings of this research in easy-to-digest terms. They relate the bacteria back to our everyday life and show the promise this research possesses.
Exposure to nature in many ways has full-body benefits especially psychologically. The hygiene hypothesis is explained here, suggesting all soil bacteria are helpful for mood benefits and our target bacteria is cited here as the main one.
Depression’s wide-reaching consequences and devastating symptoms are explained here along with the hygiene hypothesis, and the potential use for this bacteria as a vaccine is suggested.
The image included shows a blister pack of M. vaccae supplements created by Key Capital and Immunitor. Here is the website detailing this business venture.
References:
Ballenger, James C.. “Anxiety and Depression: Optimizing Treatments.” Primary care companion to the Journal of clinical psychiatry vol. 2,3 (2000): 71-79. doi:10.4088/pcc.v02n0301
Cailey Rizzo June, et al. “Scientists Are Working on a ‘Stress Vaccine’ That Could Help With PTSD and Anxiety.” Scientists Are Working on a ‘Stress Vaccine’ That Could Help With PTSD and Anxiety (Video), Travel + Leisure, 18 June 2019, https://www.travelandleisure.com/trip-ideas/yoga-wellness/researchers-developing-stress-vaccine-with-soil-bacterium
“Can Dirt Double as an Antidepressant? The Mood-Lifting Benefits of Soil Microbes.” Dave Asprey, 8 Jan. 2021, https://daveasprey.com/mood-benefits-dirt-soil-microbes/.
Limbana, Therese et al. “Gut Microbiome and Depression: How Microbes Affect the Way We Think.” Cureus vol. 12,8 e9966. 23 Aug. 2020, doi:10.7759/cureus.9966
Logan, Alan C et al. “The Microbiome and Mental Health: Looking Back, Moving Forward with Lessons from Allergic Diseases.” Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology vol. 14,2 (2016): 131-47. doi:10.9758/cpn.2016.14.2.131
Stefanie Malan-Muller, Mireia Valles-Colomer, Jeroen Raes, Christopher A. Lowry, Soraya Seedat, and Sian M.J. Hemmings.OMICS: A Journal of Integrative Biology.Feb 2018.90-107. doi:10.1089/omi.2017.0077
Spichak, Simon. “Vaccinating against Anxiety and Depression.” Medium, Invisible
Illness, 22 Oct. 2020,
https://medium.com/invisible-illness/vaccinating-against-anxiety-and-depres
sion-bfd3df324bbe.
Skonieczna-Żydecka K, Marlicz W, Misera A, Koulaouzidis A, Łoniewski I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. Journal of Clinical Medicine. 2018; 7(12):521. doi:10.3390/jcm7120521
Taylor, Valerie H.. “The microbiome and mental health: Hope or hype?.” Journal of psychiatry & neuroscience : JPN vol. 44,4 (2019): 219-222. doi:10.1503/jpn.190110
Tizenberg, Boris N., et al. “Christopher Lowry: Professor (Associate): Phd: University of Colorado Boulder, CO: Cub: Department of Integrative Physiology.” Christopher Alan Lowry, ResearchGate, 8 Oct. 2021, https://www.researchgate.net/profile/Christopher-Lowry.
Yakimovicz, Ann. “The Dirty Healthy Germ That Gardeners Love: Mycobacterium Vaccae.” Second Summer Residential Landscape Architecture, Second Summer Residential Landscape Architecture, 17 Sept. 2020, https://www.enjoysecondsummer.com/articles/the-dirty-bacterium-that-gardeners-love-mycobacterium
