Background
A unique and diverse array of inter-specific relationships can be found within the microbiome of the human gut. Due to the constant flow of microbe-carrying nutrients through it, the gut is subject to a high risk of foreign invaders. Fortunately the immune system, as well as the digestive tract (with the help of its residential microbes), have processes to rid themselves of the pathogenic strains (Ichinohe et al, 2011). These bodily systems provide a unique example of positive interactions between co-existent, and co-dependent, systems. Although the body can protect itself from damage induced by invading species, certain medical diseases such as inflammatory bowel disease (IBD) and Crohn’s disease (CD), when combined with inflammation and intestinal distress are prone to exhibit symptoms such as “severe muscle wasting and fat loss’ (Schieber et al 2015). Although the gut biome and the immune system may not always be capable of preventing these resultant health issues on their own, in conjunction they are capable of properly defending the body from muscular wasting caused by pathogenic effects.
The intestinal tract, while utilizing physical and chemical forces to break down and absorb vital nutrients, also houses a diverse community of microorganisms, a microbiota. This microbiota, which aids in increasing efficiency in digestion and absorption, is the product of coevolution and tolerance within both the host (providing this environment) and the residential microorganisms (Schieber et al 2015).
Host-microbe interactions are bound to produce negative interactions; the body has developed many defenses to foreign invaders such as microbial associated patterns (MAMPs) and host-encoded pattern recognition receptors (PRRs) (Rangan et al 2016). Both MAMPs and PRRs prompt other systems to kill the invading pathogenic microbes. Host-pathogen interactions can include defensive strategies with negative effects leading to a coevolutionary arms race. Characteristics of the microbe leads to the evolution of traits that allow the host to compensate for or overcome the detrimental impacts of microbial interactions. This prompts the possible beginning of an evolutionary response by the said pathogen, so that it may again gain the upperhand in the host-microbe relationship, thus creating a coevolutionary arms race (Daughtery & Malik 2012). As the body is constantly moving towards a more immune state, pathogens parallel this and coevolve to lapse their progress. This cycle creates an important role for the microbiota to fill- one to tip the scale in the hosts direction (Nicholson et al 2012).
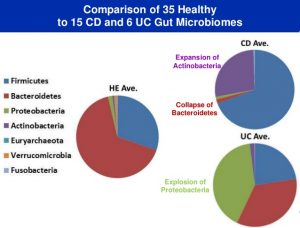
https://www.slideshare.net/Calit2LS/uci-systemsbiologyoct2013final
The intestinal tract provides a nutrient-rich, climatically constant environment for microbes (Schieber et al 2015). The diversity of the intestinal microbiota, while beneficial overall for the host, creates a battleground for the resident microbes as they compete with other residents as well as defend against invading microbes. Intestinal microbes have created a further linkage between the intestinal tract and the immune system. Not only do the bacteria provide immuno-support through elimination (via competition) of invading microbes, they are also capable of inducing not just a tolerance to certain microbes but also a form of resistance. Although substances like antibiotics can be used to control pathogens, this can disrupt the balance within the existing microbiota. Studies regarding the use of bacterial strains (that can exist in the microbiota) as a means to fight pathogenic varieties are useful as an alternative to the disruptive forces seen with the use of antibiotics.
Do resident microbes provide host protection from metabolic dysregulation induced by trauma or infection?
In a relatively new study, testing the effects of gut colonization by a strain of Escherichia coli, Schieber et al (2015) tested whether the residents of the microbiota provide the host any protection during “metabolic dysregulation’ induced by “trauma and/or infection’. In order to test whether the microbiota can produce changes in immune function and induce tolerance to certain pathogens, Schieber et al created a mouse model system with a chemically induced IBD/CD. Mice were then inoculated with the E. coli strain. Compared to test mice inoculated with heat killed microbes or no microbe, the oral administration of E. coli resulted in the colonization of control mice (but without any significant effects on weight, muscle mass, fat mass, and food consumption). The researchers also found that when mice without E. coli inoculation were infected with pathogenic strains such as Salmonella and Burkholderia, the mice experienced severe muscle wasting and fat loss, and became fatally ill. However, when mice with E. coli were infected with the same pathogens, they did not waste away as the non-E. Coli inoculated mice did.
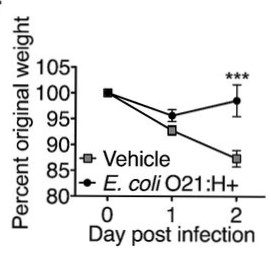
The results of their study strongly suggested that the E. Coli antagonize wasting caused by both trauma and infection within the gut, while remaining neutral when the host was in equilibrium. The presence of E. coli within the host, while having no health effects in times good health, promotes an overall trend towards wellness in times of intestinal distress.
My Questions
This field of study provides many insights into the function and control of the [human] body, yet much more data and information is needed in order to establish the regular microbe use in preventative and treatment methods as well as for medical and further scientific applications. What overall effect does our microbiota have on our health? How exactly has this symbiotic relationship influenced evolution/coevolution between the intestinal microbiota (and thus host) and its’ microbes? What possible advancements in medicine and science could lie ahead through the optimization of the benefits of a microbiota? The next steps to answer these questions lies in the research between specific microbes and their hosts. Research regarding interspecies relationships within a microbiota must be conducted which explores the vast possibilities of microbiotal makeup and the interactions between the hosts and residents. Ultimately, there lies a possibility of using the power and relationships of microbes to our advantage – to prevent diseases or lower the negative symptoms of diseases.
Want to Know more?
- The Article “Microbes Dress for Success: Tolerance or Resistance?’ provides further insight into the topic, summarizing the results of multiple studies on the topic of the microbiota. DOI: 10.1016/j.tim.2016.11.006
- The article “The gut microbiota shapes intestinal immune responses during health and disease,’ discusses the consequences of disturbing the balance of the gut microbiome on host health. DOI: 10.1038/nri2515
References
- A.M. Schieber, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science, 350 (2015), pp. 558—563. DOI: 10.1126/science.aac6468
- K.J. Rangan, et al. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science, 353 (2016) 1434—1437. DOI: 10.1126/science.aac6468
- K. Nicholson, E. Holmes, J. Kinross, R. Burcelin, G. Gibson, W. Jia, S. Pettersson. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science (2012) 1262-1267. DOI: 10.1126/science.1223813
- M.D. Daugherty, H.S. Malik. Rules of engagement: molecular insights from host-virus arms races Annu. Rev. Genet., 46 (2012) 677—700. DOI: 10.1146/annurev-genet-110711-155522
- T. Ichinohe, I. K. Pang, Y. Kumamoto, D. R. Peaper, J. H. Ho, T. S. Murray, A. Iwasaki, Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 108,(2011) 5354—5359. DOI: 10.1073/pnas.1019378108
