Background
According to the Centers for Disease Control and Prevention (2016), between the years 2000 and 2012, autism rates have risen from 1 in 150 children to 1 in 68 children diagnosed with this disorder. This disorder is most often associated with repetitive behaviors and social deficiencies and can be physically characterized by abnormal patches in the brain’s cell arrangements (Kim et al. 2017). Since autism is an increasing problem, researchers are trying to find a cause and solution to control the disorder.
Research into the human gut microbiome has led to many questions beyond what microbes can be found in the gut to how do these microbes affect a person’s whole well being? There are many beneficial bacteria within our small intestines, known as commensal bacteria, that provide protection against potentially harmful species, known as pathogenic microbes. Now researchers are trying to figure out if these microbes are capable of altering human behavior through a phenomenon known to scientists as the gut-brain axis.
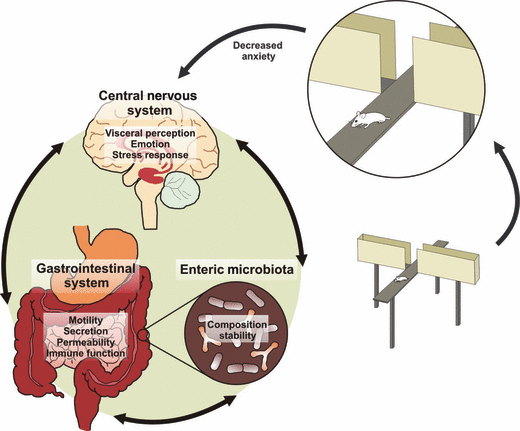
A connection has been verified between bacteria in the gut and changes in hunger through nerve signals to the brain (Konturek et al. 2004). This is an example of the gut-brain axis that points to the microbes ability to trigger physical nerves in the gut which brings a signal to the brain. Interestingly, researchers have observed possible connections between decreased gut microbial diversity and microbial richness with depression associated physical and behavioral characteristics (Kelly et al. 2016). This example of the gut-brain axis may no longer deal with directly stimulating a nerve. Instead, it could be that these microbes are producing metabolites that travel through the blood-stream, all the way to the brain. In the same realm of research, scientists are finding that the immune system’s inflammatory response may also play a role in the gut-brain axis through some unknown mechanism (Kim et al. 2016).
The cheapest option to characterize the gut microbiota of a sample is 16S rRNA sequencing. This requires a highly conserved region of bacteria’s genetic material (DNA) to be amplified. This amplified region of DNA is then run through a machine that reads each base pair of the genetic material in this region to compare to an online database that holds the identities of the bacteria that have the specific sequence. The matches are made with the variable regions which are held within the conserved regions of genetic material.
Sequencing technology has become cheaper and allowed more research to be done that involves reading of bacterial DNA. 16S rRNA amplification along with quantitative PCR (qPCR) techniques allow for many more options when viewing the human microbiome.The basic function of quantitative PCR is to amplify the section of the gene we described above and watch the number of amplifications that occur on a computer generated graph in real time. This provides researchers with a more accurate number of relative abundances of microorganisms. The research described in this article uses a combination of 16S rRNA sequencing, qPCR, and behavioral observations (defined later).
Kim et al (2017) used the new idea of microbes being part of the gut-brain axis to explore the cause of autism. They used mouse models consisting of maternal mice to answer the question below. Since mice are a commonly used model in the scientific community, the information found in a study done on mice can later be translated to humans through further research.
The Question
Do pathogenic gut microbes in mothers activate immune responses which influence the fetus’s chance of developing behavioral abnormalities that match autistic behaviors?
Evidence
The basis of this research revolves around the concept of maternal immune activation. Maternal immune activators, simplified, are anything that cause immune cells to elevate in a pregnant mouse and release inflammatory compounds called cytokines (Choi et al. 2016). (For specific compound names involved in this study, see Kim et al. 2017). There are behaviors that the offspring of mice with elevated levels of these immune cells exhibit that closely resemble autistic behaviors (Schwartzer et al. 2013). These behaviors in mice are unusual ultrasonic vocalizations, less social behavior and higher levels of self-grooming (or repetitive behavior).
Autistic behaviors were tested for, in the offspring of the pregnant mice of the various treatment groups, with a standard set of tests. A marble burying test, open field test, and social test. A marble burying test was used to characterize the level of repetitive behaviors in the mice. This test is conducted by placing a mouse in the corner of a cage with marbles and bedding. The analysis is quite simple; if the mouse buried a lot of marbles, then the mouse is said to have a high “marble burying index’ (Angoa-Pérez et al. 2013). A higher marble burying index is an indicator of autistic symptoms in mice. The open field test is meant to determine a mouse’s anxiety-like behavior which is a symptom of autism in humans. The mouse is placed in a container and the amount of time the mouse spent in the center of the container compared to the outside was measured. If the mice spent significantly less time in the center of the field than the control, the mice were determined to be exhibiting anxiety-like behavior (Bailey et al. 2009) To determine a mouse’s level of social behavior, the scientist’s used the three chamber social approach. Then the mice were placed in a three chamber cage with an inanimate object on one side and a mouse on the other. The mouse was allowed the same amount of time to explore each chamber and the observer determined how much time the mouse spent exploring the other mouse compared to the inanimate object. The more time spent exploring the other mouse, the more social the mouse is. Therefore, less social interaction in this test meant that the mouse was less social, which is a symptom of autism in humans..
To determine if the bacteria cause the inflammatory response, they treated all the maternal mice with vancomycin (an antibiotic) and then injected them with either a saline solution, for the control, or a synthetic construct that mimics a virus in the body (Kim et al, 2017). They found, if the mice were treated with vancomycin first and then the synthetic virus, the mice did not contain as many immune cells. Without as many of these immune cells present, the mice did not show autistic behaviors as tested in the mice model. This experiment suggests that removing the bacteria first makes the body’s inflammatory response to the synthetic virus lower than it would’ve been with the bacteria. Without as large of an inflammatory response, the offspring of these maternal mice did not exhibit autistic-like behaviors.
Without the antibiotic before, and only the injection of the synthetic virus, the mother mice had a higher amount of inflammatory products. Through the use of qPCR, the authors verified the vancomycin removed the bacteria responsible for the immune response, specifically single filamentous bacteria, which also contributes greatly to the presence of immune cells in the small intestine. Through the use of mice with the single filamentous bacteria and without, they found that these specific single filamentous bacteria are what elicits the immune response.
After this experiment, the researchers needed to define whether it was the exposure to the synthetic virus which led to the inflammation or if it was the presence of the bacteria in the mother’s gut that caused the autistic-like behaviors in the offspring. They found it was necessary for the bacteria to be present along with the injection of the synthetic virus during pregnancy for the offspring to exhibit abnormal behavior. This only occurred in the pregnant mice and caused the offspring to have autistic-like abnormal behaviors.
Conclusion/ Future Directions
Overall, this study suggests that the presence of bacteria which elicit an immune response in the gut of mother mice in addition to a viral infection causes the offspring to exhibit abnormal behaviors similar to behavior seen in autistic humans. If these conclusions are tested and supported in human subjects, there is the possibility of developing a drug, such as the antibiotic used to treat the mice subjects, that targets the bacteria responsible for causing an immune response. This would require more research concerning the side effects of eliminating the bacteria targeted by this drug since, as discussed earlier in this article, human gut microbiome research suggests that the microbes present in the gut are capable of both good and bad depending on numbers and diversity.
Further reading
Review paper: Human nutrition, the gut microbiome, and immune system: envisioning the future. Discusses our ability to research the gut microbiota and the need to understand how a world’s changing diet could affect our bodies. Connects nutrition, metabolism, gut microbiome and immune system.
The Gut Microbiome: A New Frontier in Autism Research A study that connects the gut microbiome with autism spectrum disorders and other neurological health.
The website Autism Speaks is a great resource to understand the struggle of having a kid with autism or to provide help to those who do. This article: Autism and Your Family is meant to help parents of a child with autism learn how to deal with the stress of the situation and be able to take care of themselves.
References
- The article discussed: Kim, S., Kim, H., Yim, Y. S., Ha, S., Atarashi, K., Tan, T. G., . . . Huh, J. R. (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. doi:10.1038/nature23910
- Konturek, S. J., Konturek, J. W., Pawlik, T., & Brzozowki, T. (2004). Brain-gut axis and its role in the control of food intake. Journal of Physiology and Pharmacology, 55(1), 137-154. Retrieved from link
- (2016). Autism Spectrum Disorder (ASD). Centers for Disease Control and Prevention. Retrieved from link
- Kelly JR., Borre Y., O’ Brien C., Patterson E., El Aidy S., Deane J., Kennedy PJ., Beers S., Scott K., Moloney G., Hoban AE., Scott L., Fitzgerald P., Ross P., Stanton C., Clarke G., Cryan JF., Dinan TG. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82:109-18. doi: 10.1016/j.jpsychires.2016.07.019
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science, Feb 26;351(6276)933-9. doi: 10.1126/science.aad0314
- Schwartzer J.J., Careaga M.,Onore C.E., Rushakoff J.A., Berman R.F., and Ashwood P. (2013). Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational Psychiatry, 3, e240. doi:10.1038/tp.2013.16
- Angoa-Pérez Marianna, Michael J. Kane, Denise I. Briggs, Dina M. Francescutti, and Donald M. Kuhn. (2013). Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. Journal of Visualized Experiments, (82) 50978. doi: 10.3791/50978
- Bailey R., Kathleen and Crawley N., Jacqueline. (2009). Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton, FL: CRC Press/Taylor & Francis.
- Cryan J.F., S.M. O’Mahoney. (2011). The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterology and Motility, 23:3 187-192. doi: 10.1111/j.1365-2982.2010.01664.x
