Background:
Much insight has been gathered about gut microbiota (the bacteria present in our bodies) and its overall role in human health, but a majority still remains unknown. Based on previous studies it is understood that gut microbiota and diversity are greatly influenced by individuals and their weight characteristics (lean and obese) (Min et al., 2019). But little to nothing is known in regards to sex-specific association, fat distribution and their effects on the bacterial gut communities. While men tend to have a lower average body fat percentage than females, the locations in which men and women store their fat could also play a great role in overall gut microbiota (Min et al., 2019) . These variations in the microbiome could possibly be linked or help explain adverse health effects seen in men and women, such as abdominal obesity in men and metabolism differences (Min et al., 2019). It is believed that the distribution of body fat on women aids in the protection in metabolic diseases such as type two diabetes and other life threatening illnesses (Karastergiou et al., 2012).
Men tend to distribute their body fat to their abdominal area and their upper body/trunk region, this is known as android fat. While females tend to distribute their fat to their hips, thighs and breasts, known as gynoid fat (figure 1.). Min et al., 2019 believes that the gut microbiome is possibly affected by fat distribution patterns seen in males and females as well as overall obesity in the two sexes based on gynoid and android fat ratios.

The central question:
Do these different distributions in fat ratios commonly found in men and women affect the bacterial communities found in the gut?
Results from the study:
Min et al., 2019 examined these patterns in fat distribution and determined that there should also be sex-specific microbiome signatures indentified, such as bacteria present specific to the fat distribution that accounts for the differences between males and females. Body composition measurements from dual x-ray absorptiometry (DXA) and gut microbiota from 16s rRNA sequencing (the process where the 16s region of a gene is specific like a barcode to particular bacteria and used to identify bacteria) were taken. DXA is a form of body composition measurement that uses an X-ray to compare and measure bone density, fat abundance and muscle mass on the human body (figure 2.). There were 212 participants whose data was taken from the WELL-China study. 45% of the participants were male and 55% were female with the mean age for both sex’s being 51.
What the study found:
Males and females were then separately categorized by their fat ratios from least to greatest. Then individually compared to determine 3 groups of bacteria made from using the total number of taxon abundance from the participants. The bacterial groups from the participants were then compared to the calculated bacterial groups from the total number of species of bacteria found from of each individual, and were found consistent with one another. Results from these tests were not significant for any of the male and female gynoid/android fat ratios. However, there were patterns observed such as in the womens android fat ratio and microbial abundance heat map; as abundance and diversity increased, high android fat ratio in females decreased while the low android fat ratio in females increased. In the male subjects a quarter of the subjects in the lowest microbiome abundance group had the highest gynoid fat ratio.
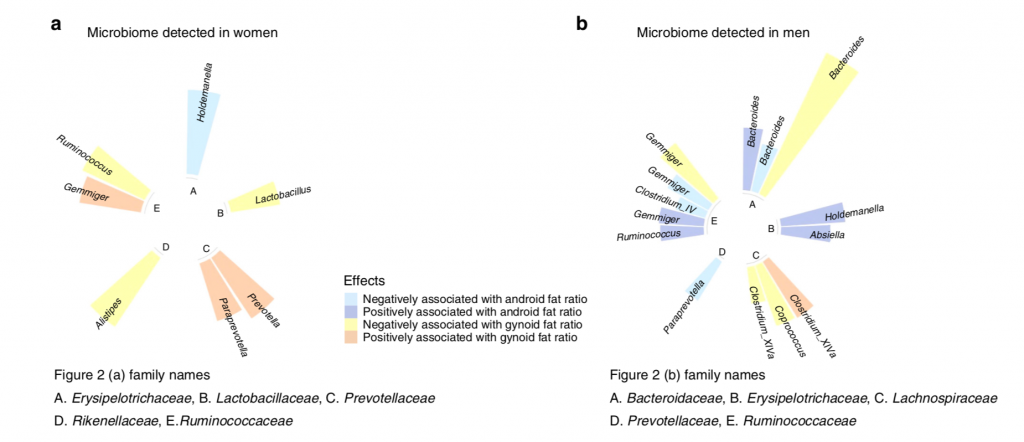
Tests were performed to test for gynoid and android fat ratios and its associated taxa by sex. In women Holdemanella was negatively associated with android fat ratio, and had no particular positively correlated bacteria for android fat ratio. Alistipes, Lactobacillus, and Ruminococcus were negatively associated with gynoid fat ratio; Prevotella, paraprevotella, and Gemmiger were positively associated with gynoid fat ratio in women (Figure 3.).
In men Gemmiger, Bacteroides, and Paraprevotella were negatively associated with android fat ratio; Gemminger, Ruminococcus, Bacteroides, Absiella, and Holdemanella were positively associated with android fat ratio. Bacteroides, Gemminger, Coprococcus and Clostridium_XIVa were negatively associated with gynoid fat ratio; Clostridium_XIVa was positively associated with gynoid fat ratio (Figure 3.).
Evidence:
While results indicate that women and men have a similar microbial abundance in association of android fat ratio, this study produced insignificant results. Possibly replicating this study with a much larger sample size could help determine statistical significance with the trends and patterns observed in this study. With the correlation between men and women who shared an increase in abundance and diversity as gynoid fat ratio decreased, it so happens the two sexs’ had different microbiomes accounting for this possible trend identified at the genus and family level.
Bacteroides, an oxygen independent spore, had no association with either female fat ratios but had an impact on both negative and positive associate in male android fat ratios. Many different taxa of bacteria accounted for different associations in the contrasting male and female fat ratios. Ruminococcus was observed to be negatively associated in women and positively associated with men in the Min et al., 2019 study. In other literature Ruminococcus have been identified as more prevalent in obese individuals opposed to those who are non-obese (Kasai et al., 2015). Bacteroidaceae and Ruminococcaceae are negatively associated with metabolic syndrome, which affects both regional adiposity and gut microbiota (Min et al., 2019). Some other underlying factors that could account for the differences in taxa observed could have been that almost half of the women participating in this study were going through menopause. Menopause causes a shift of fat towards a gynoid fat (male) distribution and away from traditional android fat distribution seen in [younger] women. (Karastergiou et al., 2012). The sex-specific microbiome signatures identified here today are a good starting point for further investigation as well. Further identification between these taxa and their larger role in the microbiome between men and women could provide valuable insight on fat distribution and its effects on the microbiome of the human gut.
My Idea/future directions:
The larger difference in men and women can be traced back to the difference in hormones and their effect on the human body. Rather than asking about the differences in fat distribution, asking how hormones and their abundances between males and females, accounts for microbial differences. Studies experimenting with mice have seen that microbiomes don’t start to differ in composition until after puberty and sex hormones become more abundant (Yurkovetskiy et al., 2013). Male mice post puberty that underwent castration, had microbiomes that changed to resemble those of the female mice post puberty which suggest hormones account for larger microbial shifts. While males after puberty (humans) see an increase in lean mass in body composition while females experience an increase in their fat mass (Karastergiou et al., 2012). I think by studying how hormones affect both the microbiome and fat distribution in both sexes, a greater understanding as to how the body works in regards to the microbiome will be achierved. Other areas of study such as how diet and overall lifestyle habits differ among men and women with “healthier’ microbiomes could be valuable knowledge.
Future Readings:
- How males and females (mice) microbiomes differ pre and post puberty and how this affects autoimmune diseases.
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400—412 (2013) doi: 10.1186/2042-6410-3-13
- How the microbiome differs based on lean and obese individuals (mice and humans) and its relationship with diet.
Clarke, S. F. et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 3, 186—202 (2012) doi: 10.4161/gmic.20168
- An article on the health differences among men and women; why women are living longer than men in relation to genetic and environmental factors. Harvard Health
References:
Karastergiou, K., Smith, S. R., Greenberg, A. S. & Fried, S. K. Sex differences in human adipose tissues–the biology of pear shape. Biol. Sex Differ. 3, 13 (2012) doi: 10.1186/2042-6410-3-13
Kasai, C. et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 15, 100 (2015) doi: 10.1186/s12876-015-0330-2
Min, Y et al. Sex Specific association between gut microbiome and fat distribution. Nature Communications. 10 (2019) doi: 10.1038/s41467-019-10440-5
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400—412 (2013) doi: 10.1186/2042-6410-3-13
