Background
There are many common bovine diseases yet there is one in particular that is undetected, difficult to treat and detrimental for farmers. Endometritis is a common bovine disease that is an inflammation of the innermost layer of the uterus. It is typically seen 21 days postpartum and there are no symptoms of illness. Endometritis occurs due to the dilating of the cervix and relaxation of the vagina; this natural occurrence post-birth allows the essential barriers to become impaired and the uterus becomes compromised (“Endometritis in Cattle”). Another reason this occurs is that the cow’s immune system is suppressed in the last few weeks of pregnancy and post-birth.
Endometritis can be split into two groups: subclinical and clinical. Subclinical is the non-severe form that is typically undiagnosed because there is no external clinical finding. Clinical is the diagnosed and treated disease; typically showing vaginal discharge. Whereas subclinical is only diagnosed through cytology.
This disease is very important when it comes to dairy farmers and leads to a great economic loss when it causes infertility (Wang et al. 2018). This is due to the time-consuming treatment, decreased reproduction, and increased chance of slaughtering the cattle. Most cattle are able to self-cure through the natural uterine endometrium healing process post-calving; however, the time it takes creates an economic loss and it negatively impacts future reproductive performance. The most prominent reason why farmers are devastated by endometritis is because it affects ovarian cycles. By affecting the ovarian cycles the thickening of the uterus does not occur, and creates a decrease in conception rate (Lee et al. 2018). Endometritis is caused by a bacterial infection in the uterus thus, finding the root microbiota that causes clinical endometritis would make a significant impact on the knowledge of why and how it occurs.
Due to economic loss endometritis in bovine is researched in order to find the root cause along with new ways to treat and possibly cure these animals. In 2018 Wang et al performed a study, “Uterine Microbiota of Dairy Cows with Clinical and Subclinical Endometritis,” was conducted on a commercial dairy farm in Beijing, China. The scientists were focused on three particular questions to study the uterine microbiota.
The Three Questions:
What microbiota is present in clinical and subclinical endometritis? What bacterial genera were most prominent? How certain bacterial interactions are associated with clinical endometritis?
Evidence:
Twenty-seven cows were used for statistical analysis for the complete data set. Thirty days post birth all twenty-seven cows underwent a vaginal inspection, rectal palpation of the uterus, cytology of the uterus lining, and had an overall health exam. Cows with clinical endometritis typically had vaginal discharge containing mucus and/or pus. Cows without vaginal discharge, signs of illness and an increased number of immune cells were categorized as subclinical endometritis. Healthy cows were classified by having clear or translucent discharge and a low number of immune cells. Samples were collected through a sterile saline aspirate inside the uterus and of the vaginal discharge. Two tubes were collected and used for cytological examination and DNA extraction.
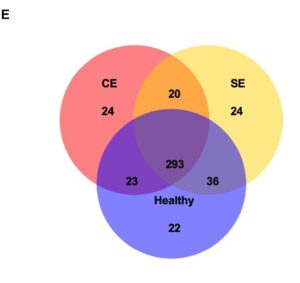
Focusing on the Venn diagram (E) this shows the shared and different microbiota between healthy, subclinical endometritis, and clinical endometritis. The uterine microbiota of healthy, subclinical, and clinical endometritis cows had similar levels of microbial diversity and shared 293 microbes. These microbes were the primary microbes that dominated the uterus. An analysis to show variation was conducted and showed that there are significant differences in the composition of microbes between healthy and clinical endometritis, while there was not a significant difference between subclinical endometritis and healthy bovine (Wang et al.).
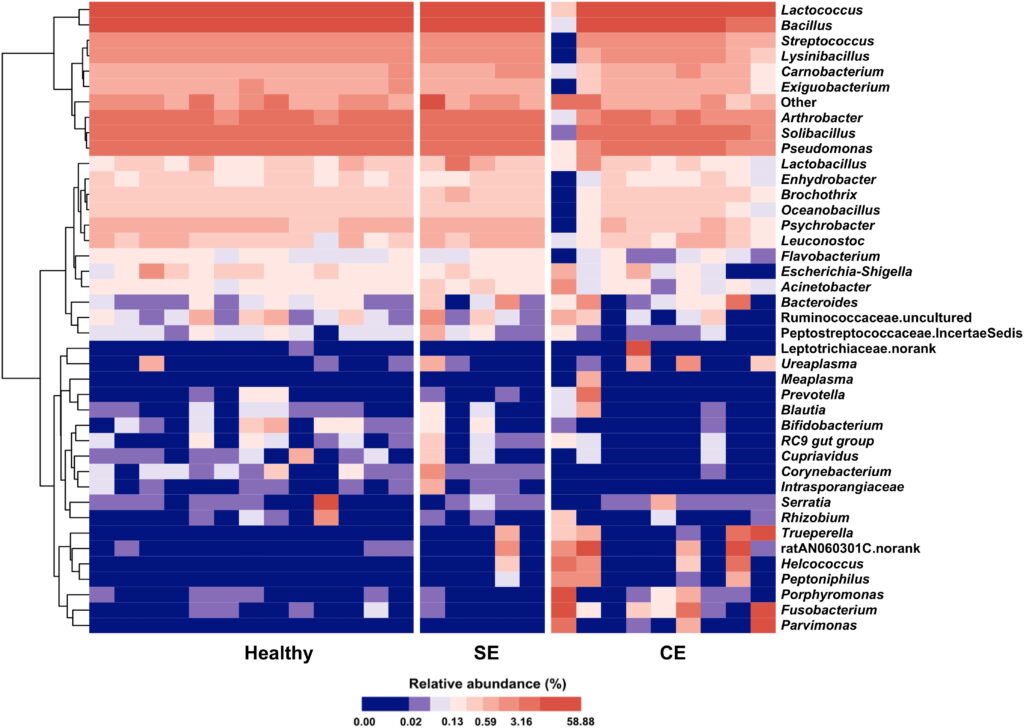
Based on these differences a total of 17 phyla were identified in the uterine microbiota. Out of these 17, five were the most abundant in clinical endometritis cattle. Supporting this finding is the heat map of the categorized microbiota (Fig. 2). Comparing healthy cows to subclinical endometritis cows on the heat map there is not a huge difference seen; however, when we compare healthy cattle to those diagnosed with clinical endometritis there is greater variation. This study showed certain bacteria were associated with clinical endometritis. LEfSe, a test that uses biomarkers to compare microbiota, distinguished the prevalent microbiota in clinical endometritis and showed that the increase of Fusobacterium, Trueperella, and Peptoniphilus were the most enriched and impactful on the uterus (Wang et al.). With this in mind based on co-occurrence grouping, which finds relationships between microbes, it is likely that Fusobacterium is mutualistic with Trueperella, Porphyromonas, Parvimonas and other bacteria which will cause an imbalance in the uterus causing clinical endometritis (Wang et al.). However, in subclinical cattle, the information supported that major pathogens played a minor role in bovine uterine infections.
My Questions?
The next research I would like to see is how could we prevent endometritis from happening? From this study, we know what microbiota cause endometritis and I think a study to prevent the onset of endometritis in cows could be done pretty easily. The way this study could be accomplished by taking samples of cows diagnosed with subclinical and clinical endometritis. Then growing the causative bacteria on petri dishes or in an environment they would be able to grow in. Different antibiotics or probiotics would then be incorporated to see which is most effective against all of the bacteria. Understanding which antibiotic/probiotic is the most effective would allow farmers to incorporate it into the cows’ diet and help prevent endometritis postpartum. This also brings up more questions. Is there an antibiotic that would cause the milk to become unusable? How would this pretreatment possibly affect the calf? How would it affect the cow’s uterine microbiota?
Further Reading
A video showing the diagnosis of endometritis using an ultrasound by Keebo Vet.
Here’s another video showing a case presentation on the diagnosis and clinical management of endometritis in cattle.
This source provides basic background information on bovine and equine endometritis.
This review entails all the risk factors endometritis may cause in cattle postpartum.
References
- Wang, Meng-Ling, et al. “Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis.” Frontiers, Frontiers, 22 Oct. 2018. doi: 10.3389/fmicb.2018.02691
- Lee, Soo Chan, et. al. “Cytological endometritis in dairy cows: diagnostic threshold, risk factors, and impact on reproductive performance.” US National Library of Medicine, National Institutes of Health, 23 March 2018. doi: 10.4142/jvs.2018.19.2.301
- “Endometritis in Cattle.” Vet360, 22 Sept. 2016,
- Gilbert, Robert O. “Metritis and Endometritis in Large Animals – Reproductive System.” Merck Veterinary Manual, Merck Veterinary Manual, 2015.
- “Common Bovine Diseases.” Veterian Key, 11 Aug. 2016.

