Background
Microscopic organisms, microorganisms, and microbes for short, are all terms that encompass all organisms too small to see with the naked eye. Microbes, being as small as they are, pretty much keep the planet running. Some important functions of microbes on a planetary scale include breaking down pollutants, ensuring that the soil is fertile, and powering the Earth’s biogeochemical cycles (Falkowski et al 2008). We need all these external microbes to live, but we also need microbes in and on our bodies to live. You can view the human body as a planet and the microbes being the inhabitants. Just like the planet earth, the human body also has varying climates where certain species thrive. Microbial communities in the human body have their own community structure and function (Human Microbiome Project Consortium 2012).
The bacterial cell to human cell ratio in any person is about 1:1. This means that your body is just as much bacteria as it is you. (Sender et al 2016). This ratio doesn’t include other microorganisms that aren’t bacteria, so in reality, microbial cells easily outnumber human cells. Studies show that this population of microbes possibly starts in utero (Collado 2016), but the significant event of exposure is during birth (Mändar, R., & Mikelsaar, M. 1996). Researchers believe that understanding how the human microbiome is established and maintained in the first stage of life is essential to understanding the role of microbes in human health later in life.
Central questions
With the growing popularity in cesarean deliveries, it is important to understand the effects of cesarean and vaginal deliveries. Does mode of delivery have any effect on the infant’s microbiome? If so, how long does it last? The authors of a study published in 2017 also had these same questions (Chu et al.) The study focused on the microbial communities present at each type of birth and then tested infants six weeks following birth to see if any differences were still present.
What did they find?
Using two methods of characterizing the microbiota, whole-genome shotgun sequencing and 16S rRNA sequence analysis, the authors investigated the composition and function of the microbial communities for both the newborns and mothers at the time of delivery and 6 weeks after.
A primary characteristic of the adult microbiome is body site differentiation. Analysis comparing the neonatal microbiota body sites shows that at the time of delivery, there is no significant body site differentiation. However, at 6 weeks of age, infants showed body site-specificity, which means that each microbial community is specific to each body site. The difference in microbes between the infants at birth and after 6 weeks can be visualized by the following two figures (Fig 1c. and Fig 2c. from Chu et al.) The figures both show average relative abundances represented by the size of the circles. The indicator value index represents the specificity of that taxon to that particular body site.
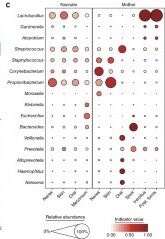
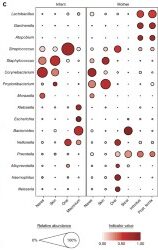
The reorganization of the infant microbiota after six weeks is due to the fact that each body site now has different conditions than they did at birth, where, up until then, the entire body was surrounded by amniotic fluid and the conditions were equal in each site. Outside of the mother, different areas of the body hold varying amounts of moisture, oxygen, and sources of nutrients for the microbes.
The authors investigated the mode of delivery and its effect on the infant microbiome. The results of a SourceTracker analysis showed that vaginally birthed infants are populated by vaginal and skin microbiota from the mother, where unlabored cesarean-delivered infants were mostly populated by the microbiota found on the mother’s skin. This makes sense because the baby has no contact with the vast amounts of microbes in the birth canal.
Similar to the body-site specificity study, after six weeks, reorganization of microbes occurred with the infants microbiome and there was no significant difference between the infants that were vaginally birthed and the ones that were cesarean-delivered. After six weeks, all significant differences between the modes of delivery had disappeared.
Keeping in mind the amount of work that had to be done for this study, some people might think that because the authors didn’t find a difference, the findings aren’t significant enough. From my view, I see that we now know that, in terms of the microbiome, cesarean-delivered babies aren’t at a disadvantage compared to vaginally-birthed babies. From my perspective, the authors of this paper described and justified their methods for the study well enough that I wasn’t left with any doubts or questions about the experiment.
Evaluation and further research possibilities
The researchers initially had 82 mother-infant pairs to sample, but realized they needed an even larger sample size to detect the difference they were looking for, so they DOUBLED the sample size for the samples at birth. However, only the original sample pairs were kept to study after the six week (n=60 due to loss in participation. . .but still a large number.) I appreciate the sheer volume of this entire experiment to fill this gap in knowledge.
Another aspect of this experiment that I have come to appreciate is that whole-genome shotgun sequencing was used in addition to 16S rRNA sequencing analysis for every sample. Many studies tend to use 16S rRNA because it is practical and less expensive than whole genome sequencing. Whole genome sequencing (WGS) offers insight into the total microbial community, however, WGS requires much more data analysis.
There were several factors brought up that could contribute to differences in microbiomes in infants such as feeding types (breastfed, formula-fed, or combination), diet types of the mother, and physical condition of the mother. Personally, I would be interested in using this data to further a study based on feeding types. Being a mother myself, I hear arguments about “breast is best”, so naturally I am interested in seeing a longitudinal study with this sample size on the microbiomes of children and if breastfeeding children instead of formula feeding has any lasting benefits.
Additional Reading & Videos:
Microbes from Mom: Vaginal Birth vs. C-Section from the I Contain Multitudes youtube channel gives an overview of the basics of this paper and also an insight to a study about wiping down cesarean-delivered babies with towels that were kept in the vaginal canal and how this might mimic being vaginally birthed.
If you’re interested in the role that the physical condition of the mother plays in the microbiota of their infants, The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD by Soderborg et al. is a good place to start.
References
- Chu, D. M., Ma, J., Prince, A. L., Antony, K. M., Seferovic, M. D., & Aagaard, K. M. (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature medicine, 23(3), 314–326. doi: 10.1038/nm.4272
- Collado, M. C., Rautava, S., Aakko, J., Isolauri, E., & Salminen, S. (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific reports, 6, 23129. doi:10.1038/srep23129
- Falkowski, P. G., Fenchel, T., & Delong, E. F. (2008). The microbial engines that drive Earth’s biogeochemical cycles. Science, 320(5879), 1034-1039. doi:10.1126/science.1153213
- Human Microbiome Project Consortium. (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi:10.1038/nature11234
- Mändar, R., & Mikelsaar, M. (1996). Transmission of mother’s microflora to the newborn at birth. Neonatology, 69(1), 30-35. doi: 10.1159/000244275
- Sender, R., Fuchs, S., & Milo, R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS biology, 14(8), e1002533. doi:10.1371/journal.pbio.1002533.
