
BACKGROUND
Picture this, you are a field researcher who stumbles upon a population of diseased and mutated humans. These humans live in an environment plagued with disease and pollution. The question you have is whether the mutation in the humans caused the disease/pollution or if the disease/pollution caused the humans to mutate. This question forms the basis of what microbiologists are interested in today; is the gut microbiome causal in the formation of [autoimmune] diseases, or is the microbiota the result of the diseased environment in which they inhabit?
When referring to the bacteria and other little critters that live on and in our body (a.k.a. microbes), we often use the term “microbiota’. While the term “microbiota’ simply refers to just the microbial inhabitants, the term “microbiome’ encompasses both the microbial inhabitants as well as the environment in which they live .
Multiple Sclerosis (MS) is an autoimmune disease, or a disease in which the body attacks itself. While we know MS is an autoimmune disease, the origin of the disease is not well understood and scientists still do not know whether it stems from the central or peripheral nervous system. Although unaware of the autoimmune mechanisms at play, there is a disease concordance of 25% in monozygotic twins (if one identical twin were to have MS, there is a 25% chance the other twin has MS) indicating that both environmental and genetic factors contribute to the development of MS. The microbiome may be a vital environmental factor when analyzing cause of disease. The gut microbiome has been shown to be associated with “obesity, digestive, endocrine, inflammatory, and autoimmune disorders,’ although the mechanisms connecting these diseases and the microbiome is not yet fully understood (Swidinski 2017). The contents of the gut microbiome helps to guide future immune responses by exposing the body to antigenic markers (McDermott 2014). The body is exposed to antigenic markers via the contents of the gut microbiome, which helps to guide future immune responses to antigenic threats. Previous studies have shown that human autoimmune diseases are often associated with an altered commensal microbiota, or the helpful antigen-fighting bacteria that are typical of the gut microbiome (Kostic 2014). Researchers also found a decrease in bacteria associated with proinflammatory conditions within the guts of those with MS (Scher 2013) . In order to better understand the connection between the microbiome and MS, researchers used a relapsing-remitting MS mouse model with spontaneous experimental autoimmune encephalomyelitis (EAE), the mouse equivalent of human-MS (Berer 2011). The mice were raised in a germ-free environment; germ-free environments typically result in immunocompromised mice, when bacteria are introduced to the gut microbiome the effects modulate the development and severity of EAE (simulating the effects of MS). When mice were inoculated with commensal microbes (without pathogenic microbes), they began to express symptoms of EAE, indicating a connection between the cause/symptoms of diseases and the gut microbiome.
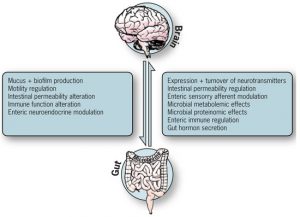
CENTRAL QUESTION
Because the gut microbiome plays a vital role in immune function and autoimmune diseases, the researchers in this study (Jangi 2015) wanted to look at the contents of the gut microbiota in individuals with MS in order to identify alterations in the gut microbiota and find correlations with MS-associated immune changes and treatment using immunomodulatory therapy(immunomodulatory therapy is a medical treatment used to suppress immune responses).
EVIDENCE
Researchers sampled the gut microbiome of 60 individuals with MS, and 43 healthy control individuals whose demographics were similar to those of the test subjects. Of the 60 subjects with MS, 28 individuals were not receiving any immunomodulatory treatments. The researchers (Jangi 2015) found there was no significant difference in microbial community structure between the two groups. When looking at beta diversity, beta diversity compares diversity between test subjects and between test groups (MS vs Control), they found no significant community differences. Although they did not find any significant differences in beta dsity of overall community structure, they did find differences in the relative abundances of the microbiota at the phylum level. Subjects with MS were found to have increased abundances of the phyla Euryarchaeota and Verrucomicrobia compared to healthy controls. Within these phyla, they found increased abundances of genera Methanobrevibacter(Euryarchaeota) and Akkermansia (Verrucomicrobia). They also found a genus, Butyricimonas (phylum Bacteroidetes), at decreased abundances in both treated and untreated subjects. Medicinal treatment may often times alter the contents of the microbiome, which was found to be true in this study. Genera Collinsella and Slackia (phylum Actinobacteria) and Prevotella (phylum Bacteroidetes) were found in decreased abundances within the untreated MS patients, indicating these bacteria may be associated with the diseased state since both treated and control individuals had increased abundances of these genera. While treatment may “normalize’ some of the MS-related changes in the gut microbiome, a decrease in genus Sarcina only in treated subjects suggests there may also be negative treatment-associated effects on the gut microbiome.
Researchers also assessed the sera (all blood proteins besides clotting factors; serum includes antibody proteins) from both test groups; although the MS group had an increased abundance of Methanobrevibacter, there was no difference in antibody titres between the two test groups (Bang 2014). Methanobrevibacter is a methane-producing microbe. Researchers found an increased level of methane in the breath of subjects with MS than in the controls (Mckay, 1985). In this study (Jangi 2015), testing breathe methane levels was done on a different cohort of test subjects, independent from the original test subjects. The MS cohort in this group was represented by MS patients with varying levels of gut methanogens, and while this test group is representative of a general population of MS patients, it would have been nice if this part of the study could have been done with the same test subjects as the first part of the study in order to ensure consistent results and to be able to more directly correlate the results of both parts of the study to each other.
MY REMAINING QUESTIONS
While we know patients with MS have increased abundances of Euryarchaeota and Verrucomicrobia, we don’t know exactly what roles these phyla have in the healthy/diseased state of MS. Do these phyla exacerbate MS symptoms? Or are these phyla simply a result of a diseased microbiota? The average length of disease duration in the test subjects was 12.8 ±8.3 years. Perhaps a study comparing the abundances of phyla like Euryarchaeota and Verrucomicrobia throughout the course of the disease, either by comparing individuals with disease durations of 1, 2, 5 etc. years or sampling individual patients over the course of their disease, would provide valuable insight into the influence of Euryarchaeota and Verrucomicrobia by finding a trend in the overall abundances. If there is a correlation between Euryarchaeota and/or Verrucomicrobia and MS disease progression, we could test how changing/normalizing these abundances stears disease progression.
Can a healthy-type (non-diseased) microbiome alter the presentation of M.S. symptoms? A next step in understanding the relationship between the microbiome and autoimmune diseases like MS would be to look at the effect of transplanting a healthy (non-diseased) microbiome in someone with MS through the use of either probiotics or fecal transplants. If results from the introduction to a more normalized microbiota were to show a decrease/alteration in MS-related symptomatology, more work could be done in determining how to shape the MS microbiota to prevent MS symptoms. This could further lead to vital information regarding the mechanisms at play in the development of MS.
FURTHER READINGS
In a follow up study, researchers analyzed the effects of a ketogenic diet on the expressed symptoms of MS. This study shows the impact of diet on a diseased model.
Swidsinski, Alexander et al. “Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet.’ Frontiers in Microbiology 8 (2017): 1141. PMC. Web. 6 Oct. 2017. DOI: 10.3389/fmicb.2017.01141
The point of learning how the body interacts with the microbiota is to eventually use this information to create medicines that take both human and microbiota genomes into account.
Mai V., Prosperi M., Yaghjyan L. (2016). Moving microbiota research toward establishing causal associations that represent viable targets for effective public health interventions. Ann. Epidemiol. 26, 306—310 DOI: 10.3389/fmicb.2017.01141
REFERENCES
- Bang, C., Weidenbach, K., Gutsmann, T., Heine, H. & Schmitz, R. A. The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PLoS ONE 9, e99411 (2014). doi: 10.1371/journal.pone.0099411
- Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538—541 (2011). doi: 10.1038/nature10554
- Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016). doi: 10.1038/ncomms12015
- Kostic, A. D., Xavier, R. J. & Gevers, D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146, 1489—1499 (2014). doi: 0.1053/j.gastro.2014.02.009
- McDermott, A. J. & Huffnagle, G. B. The microbiome and regulation of mucosal immunity. Immunology 142, 24—31 (2014). doi: 10.1111/imm.12231
- McKay, L. F., Eastwood, M. A. & Brydon, W. G. Methane excretion in man–a study of breath, flatus, and faeces. Gut 26, 69—74 (1985). https://www.ncbi.nlm.nih.gov/pubmed/3965369
- Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202 (2013). doi: 10.7554/eLife.01202
- Swidsinski, Alexander et al. “Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet.’ Frontiers in Microbiology 8 (2017): 1141. PMC. Web. 6 Oct. 2017. doi: 10.3389/fmicb.2017.01141
