Background
Depression is a mood disorder that is heterogeneous in nature. Depression causes severe symptoms that affect how a person feels, thinks, and handles daily activities (NIMH, 2017). According to the World Health Organization, depression affects over 300 million people and is a major worldwide contributor to the burden of diseases. This is especially pertinent considering that depression is one of the mood disorders associated with suicide, some others being anxiety, schizophrenia and PTSD. On an annual basis suicide leads to the death of nearly 800,000 people and is the second leading cause of death within the age group of 15 to 29 year olds (WHO, 2017). The underlying causes of depression are a complex interaction of social, psychological, and biological factors. It is essential to analyze these factors to understand the contribution of each in the development and maintenance of major depressive disorders.
When thinking of possible biological mechanisms that contribute to depressive disoders, gut microbiota, the microbes such as bacteria and viruses that occupy the gut, may not be the first thought that comes to mind. However, a study by Kelly et al. 2016 entitled “Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat’ suggests that gut microbiota have an intimate interaction with the neuroimmune, neuroendocrine, and neural pathways that contribute to the complex etiology of major depressive disorders. It is recognized that the improper regulation of these various pathways are some of the many biological drivers of mental disorders (Stetler and Miller, 2011; Dowlati et al., 2010; Berton and Nestler, 2006) which is why examining the effect of the microbiota on these neurobiological systems is imperative in understanding the pathophysiology of the disorder.
Central Question
The central question of the paper by Kelly et al. 2016 is to classify the extent by which changes in the gut microbiota composition and function mediate improper regulation of the neurobiological pathways discussed above (neuroimmnune, neuroendocrine, etc).
The authors address this central question through two methods:
- First the authors compared the microbiota of healthy people to that of depressed people by collecting fecal samples from depressed patients and healthy people matched to these patients by age, gender and ethnicity. They then use the microbial DNA found in these samples with a method known of 16S rRNA sequencing, which is an effective means of classifying what kinds of bacteria are present.
- Secondly, the authors analyzed proteins associated with neural pathways such as pro-inflammatory proteins, short chain fatty acids, salivary cortisol, kynurenine/tryptophan metabolites, C-reactive proteins, and lipopolysaccharide binding proteins in both human and mice models to see if they had any association with changes in microbiota. The mice models were generated by treating mice with a cocktail of antibiotics for 28 consecutive days to clear them of gut bacteria. Three days after the antibiotic treatment each mouse ingested donor microbiota of the fecal samples from the three most depressed people or from their matched healthy person with the intent that these microbes would colonize the gut. Each group of mice, healthy versus depressed, were then tested both behaviorally and metabolically.
Evidence
Decreased gut microbiota diversity and richness was found to be associated with depression, as shown in figure 1. Furthermore, fecal microbiota transplantation from depressed patients to the mice models was effectively able to induce physiological changes, such as altered tryptophan metabolism, and behavioral changes representative of depression. The authors argue that these results are suggestive of the causal role microbiota play in the development of depression. This is significant because if microbiota truly have a causal role in the development of depression, manipulation of the microbiota could be a method of treatment as well as a preventive measure for depressive disorders.

Some more of the physiological changes that were found to be significantly different in the human cohorts are as follows. There was found to be an increased level of pro-inflammatory cytokines in the depressed cohort compared to the healthy. These inflammatory proteins can cross the blood-brain barrier and may affect neural responses that contribute to psychiatric disorders like depression (Yarlagadda et al.,2009; Kronfol et al., 2000). However, the mouse models with transplanted microbes did not have significantly different levels of cytokines, indicative of some other confounding factor in people (e.g. smoking or alcohol consumption) rather than a microbial contribution.
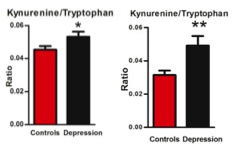
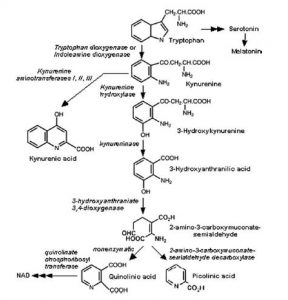
Based on the broad body of evidence suggesting tryptophan’s role in neural modulation, this study felt it was essential to analyze tryptophan metabolism’s association with the fecal microbiota of depressed patients. The researchers found that the kynurenine/tryptophan ratio was significantly different in both mice and human models, with a higher ratio in depressed people and depressed fecal matter recipient mice compared to the healthy human and mice cohorts (figure 2). This is an important finding because the transplanted microbial communities were the only difference between the groups of mice, which is indicative of the influence of microbiota on the tryptophan and kynurenine pathway. Tryptophan is an essential amino acid precursor to kynurenine and other detrimental metabolites, such as QUIN, that come from the kynurenine pathway which may contribute to psychiatric disorders (Schwarcz et al., 2012; Davis et al., 2015). Tryptophan is also a precursor to some neurologically active compounds that have beneficial neurological functions such as serotonin, which is thought to modulate mood and emotions, and melatonin, which helps control sleep-wake cycles. It was proposed that if tryptophan commits to the kynurenine pathway than there is less available tryptophan for production of beneficial serotonin, however this was proven fallacious. Although tryptophan committing to the kynurenine pathway didn’t decrease tryptophan levels in the cerebrospinal fluid, it was shown to increase inflammation through the production of kynurenine pathway metabolites which can have negative effects on neural pathways (Davis et al., 2015).
Evidence from behavioral tests also support the contribution of gut microbes in the induction of depression. In three out of the four behavioral tests, the depressed rats showed anxiety-like and anhedonia behaviors associated with depression that were significant. Although some of the variables the researchers looked into, such as proinflammatory proteins, were not found to be significantly different it is still important to delve into these pathways to see if there are any potential connections.
My questions:
The question as to if changes in gut microbiota actually plays a causal role in depression may have not been fully answered by this paper, but it leads one to wonder why some of the connections were observed. Therefore this study is a great doorway into future studies. It shows that there are some connections between gut microbiota and major depressive behaviors that are in fact transferrable and should be looked into further. For instance, recall that the authors found a higher ratio of kynurenine/tryptophan in depressive compared to healthy humans and mice. This observation leads me to wonder about the relationship between microbes and this specific pathway. Furthermore, studies could be conducted on the major types and abundances of bacteria in depressive versus healthy individuals that look at their major metabolic functions and how this may relate to chemical differences in depressed versus healthy individuals.
If we find that certain microbes play particular causal roles in neural pathways then it would be a suitable idea to look into microbial treatments for modulating depression, such as pre and probiotics. Treatments like this cannot gain any ground without controlled and experimental studies such as Kelly et al. 2016 contributing to the body of knowledge about the influence of microbes on various diseases.
Some additional readings that may be helpful in understanding some of the concepts discussed are as follows:
Additional information on the tryptophan and kynurenine pathways:
1–Schwarcz, R., Bruno, J. P., Muchowski, P. J., & Wu, H.-Q. (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nature Reviews. Neuroscience, 13(7), 465—477. doi:10.1038/nrn3257
2-Davis, I., & Liu, A. (2015). What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert Review of Neurotherapeutics, 15(7), 719—721. doi:10.1586/14737175.2015.1049999
Information on pro-inflammatory cytokines:
1-Banks, W.A. (2005) Blood-Brain Barrier Transport of Cytokines: A Mechanism for Neuropathology. Current Pharmaceutical Design, 11(8). doi:10.2174/1381612053381684
2-Banks, W. A., Kastin, A. J., & Broadwell, R. D. (1995). Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation, 2(4).PMID: 8963753
About the mouse behavioral tests conducted:
1- Hoffman, K.L. (2015) 3 — Modeling disorders of fear and anxiety in animals. Modeling Neuropsychiatric Disorders in Laboratory Animals. doi:10.1016/B978-0-08-100099-1.00003-0
2- Bogucka-Bonikowska, A., Baran-Furga, H., Chmielewska, K., Habrat, B., Scinska, A., Kukwa, A., Koros, E., Kostowski, W., Polanowska, E. and Bienkowski, P. (2002). Taste function in methadone-maintained opioid-dependent men. Drug Alcohol Depend 68(1), 113-117. doi:10.21769/BioProtoc.1822
3- Gould T.D., Dao D.T., Kovacsics C.E. (2009) The Open Field Test. Mood and Anxiety Related Phenotypes in Mice. Neuromethods, 4(na). doi:10.1007/978-1-60761-303-9_1
4- Yankelevitch-Yahav, R., Franko, M., Huly, A., & Doron, R. (2015). The Forced Swim Test as a Model of Depressive-like Behavior. Journal of Visualized Experiments’¯: JoVE, (97), 52587. Advance online publication. doi:10.3791/52587
References
- Berton, O., Nestler, E.J., (2006). New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 7 (2), 137-151. doi:10.1038/nrn1846
- Davis, I., & Liu, A. (2015). What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert Review of Neurotherapeutics, 15(7), 719—721. doi:10.1586/14737175.2015.1049999
- Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E.K., Lanctot, K.L.,(2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67 (5),446-457. doi:10.1016/j.biopsych.2009.09.033
- El Aidy, S., Derrien, M., Aardema, R., Hooiveld, G., Richards, S.E., Dane, A., Dekker, J.,Vreeken, R., Levenez, F., Dore, J., Zoetendal, E.G., van Baarlen, P., Kleerebezem, M., (2014). Transient inflammatory-like state and microbial dysbiosis are pivotal in establishment of mucosal homeostasis during colonisation of germ-free mice. Benef. Microbes 5 (1), 67-77. doi:10.3920/BM2013.0018
- Kelley, R. John, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research 82(na), 109-118. doi:10.1016/j.jpsychires.2016.07.01.19.
- Kronfol, Z., Remick, DG. (2000). Cytokines and the brain: implications for clinical psychiatry. American Journal of Psychiatry, 157(5), 683-94. doi:10.1176/appi.ajp.157.5.683
- O’Connor, J.C., Lawson, M.A., Andre, C., Moreau, M., Lestage, J., Castanon, N.,Kelley, K.W., Dantzer, R., (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14 (5), 511-522. doi: 10.1038/sj.mp.4002148
- Schwarcz, R., Bruno, J.P., Muchowski, P.J., Wu, H.Q.,(2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13 (7), 465-477. doi: 10.1038/nrn3257
- Stetler, C., Miller, G.E., (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73 (2), 114-126. doi:10.1097/PSY.0b013e31820ad12b
- Yarlagadda, A., Alfson, E., & Clayton, A. H. (2009). The Blood Brain Barrier and the Role of Cytokines in Neuropsychiatry. Psychiatry (Edgmont), 6(11), 18—22. PMCID:PMC2801483
