Background
We live with an astonishing number of microbes in our bodies. Recently, scientists have been finding increasing evidence that one’s microbial profile (the set of different types of bacteria within their bodies) is shaped at birth, often ‘inherited’ from a mother when babies pass through the birth canal. One’s microbial profile, along with the conditions within one’s body that create an environment that supports the growth of some bacteria and prevents the growth of others, is called microbiota. Its development and stabilisation co-occurs with the development of a child’s nervous system; abnormal microbiota development is suggested to cause disruption in later cognition and behaviour (Diaz et. al, 2016).
The different areas of the brain are specialised in different functions, but overall the main ‘bodies’ of brain cells- neurons– are found in the outer layers. They are called gray matter. The larger the volume of gray matter in a brain region, the more neurons are present there. The neurons have “arms’ that connect one neuron to others; these allow one neuron to be involved in circuits localised in different regions in the brain. These “arms’ are axons. They make up the white matter and facilitate communication via electrical signals. Myelin, a coating of fat, insulates the axons and speeds up the transmission of signals. Thus, more myelination in white matter is indicative of faster transmission and hence quicker mental processing. Communication within the brain also relies on a chemical system: neurons release substances called neurotransmitters that affect brain activity.
Some gut microbes are suggested to regulate the release of cortisol, the “stress neurotransmitter’, by secreting other substances that communicate with the hypothalamus, which controls most of our “instinctive behaviours’ such as eating or the way we respond to stress (Wall et. al, 2014). But so far, these studies have been focused on monitoring the levels of neurotransmitters and other substances, while actual visualisation of the process by which microbes change brain development has not been conducted.
A study done last year boasts the first time techniques for monitoring and imaging the brain in a living organism have been used to visualise changes in brain structure in association with microbial profiles.
Central Question
Researchers wanted to know: Do gut bacteria affect the development of brain structures and their behavioral functions?
Evidence
To answer this, they examined differences in brain structure development and behaviour between specific-pathogen free (SPF) mice (mice that had ‘bad’ bacteria excluded) and “germ-free’ (GF) mice (mice that did not acquire most of their essential microbes because they were conceived through in-vitro fertilisation instead of natural birth). Hence, SPF mice are used to model humans with healthy microbial community, and GF mice used to model humans in which microbial community development is disrupted.
You might have heard of MRI (magnetic resonance imaging) before. It is a machine that generally uses a large magnet rotated around a person’s head to scan their brain through the skull. Areas with higher liquid content or fat content distort the magnetic field as it passes through someone’s brain, and this distortion is picked up by the sensor, so these areas appear brighter on the image produced. The researchers of this study used macromolecular proton fraction (MPF) mapping, which is a relatively new MRI technique utilising this same idea. Because myelin is essentially a coating of fat around neurons, brain areas with more myelination will show up brighter on an MPF scan. To control for sex-specific differences and to observe brain development over time, they split the data for comparison as such: male compared to female mice, and juvenile mice compared with adult mice. Mice in the ‘juvenile’ group were tested at 4 weeks old, and mice in the ‘adult’ group were 12 weeks old when tested.
The live-body MRI found relative differences in gray matter volume of parts of the brain dedicated to different functions.
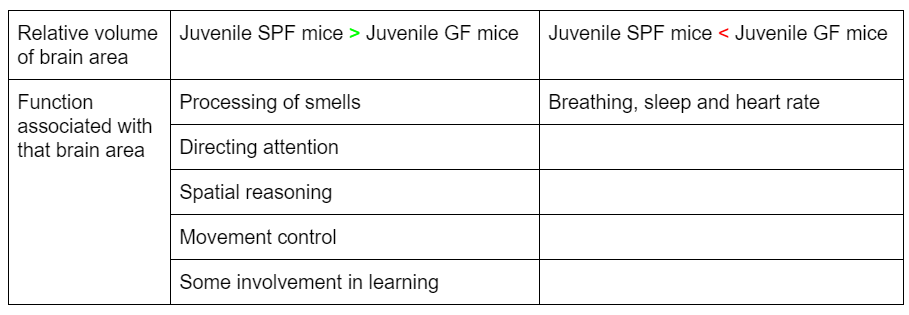
What’s particularly interesting, is that the area that had smaller gray matter volume in juvenile SPF mice compared to GF mice, was involved in more ‘basal’ functions like breathing, whereas the areas that were bigger in juvenile SPF mice are associated with ‘higher’ brain functions.
When tested with the Morris water maze, which trains mice to locate a platform within a tank of water, adult SPF mice were better at remembering where the platform was than adult GF mice, and in particular the males performed better.
The fear conditioning tests that conditioned mice to associate a noise with an electric shock showed that SPF mice showed greater fear response than GF mice at 12 weeks, especially for male SPF mice. This suggested that SPF mice, and especially male mice had better long-term memory.
They put the mice through a two-stage social interaction test involving a three-chamber enclosure set up as in the figure below.
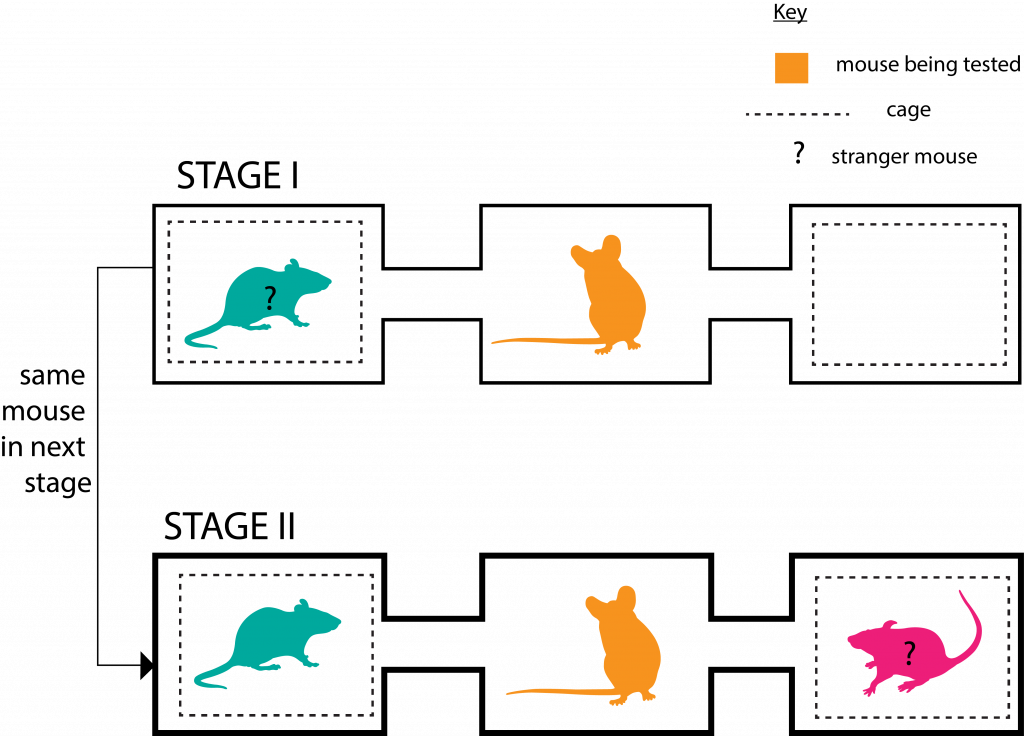
In each stage, the mouse being tested was allowed to roam the enclosure freely for 10 minutes.
In Stage II the adult SPF mice (of both genders) spent significantly more time with the new stranger mouse than their acquaintance from 10 minutes prior (of Stage I); which points to better social memory and hence novelty preference, compared to the adult GF mice.
What’s interesting is that these results concur with sexual differences in spatial memory and long-term memory in humans and other animal studies (Postma et. al 2004).
After the mice were anaesthetised, killed and had their brains sectioned, two types of higher-resolution MRI was performed on their brain sections.
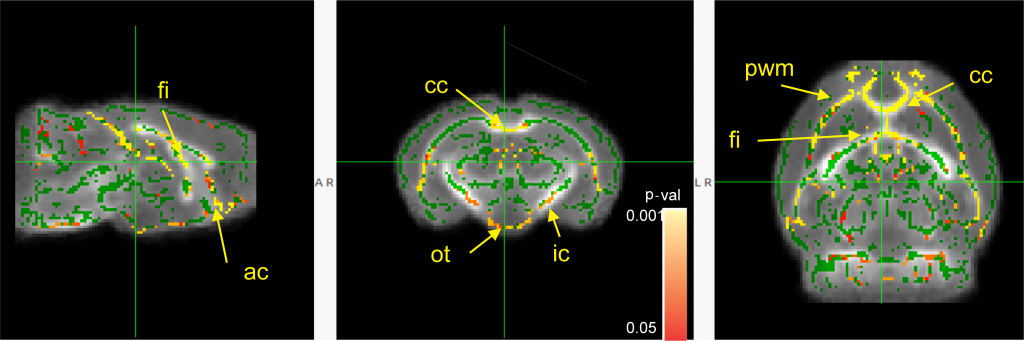
There was a significant increase in myelination in the white matter of the ‘juvenile’ SPF mice (compared to juvenile GF mice) in:
- The two bundles of axons that facilitate communication between left and right halves of the brain (cc and ac)
- Part of a region correlated with recall memory (fi)
- An axon bundle connecting to the brain region that controls movement (ic)
- The axon bundle that sends visual information from the eyes (ot)
- The layer of brain tissue around the fluid-filled space in the brain (pwm) This area is particularly important because it is where new brain cells are formed before they move to the outer layers where they are needed for continued brain function (Simon, 2017).
But there was not much difference found between the two adult groups.
The brain sections were also stained with dyes that would highlight myelin, and it confirmed that there was significantly more myelin in 3 of the areas listed above (ac, cc and ic) of the juvenile SPF mice. The SPF adult mice had more myelination than the adult GF mice, but only in the ic.
These structural differences between mice that grew up with a ‘complete’ microbe community and those that did not , confirmed to a certain extent what the researchers proposed- gut microbes influenced early brain development, such that there were observable differences in brain morphology. These differing development patterns may have contributed to better performance on cognitive behavioural tasks such as the Morris water maze, and the fear conditioning.
But why were there few differences between the adult groups? Did these differences disappear over time?
Previous studies have shown that there are changes with respect to volume in both grey matter and white matter between the phase of childhood to adolescence, but there is relatively stable total brain volume. This means that volumetric changes in certain parts of the juvenile brain does not equate to larger brain volume as adults. Rather, it might be that the advanced development in these parts lead to advanced development in cognitive faculties , even if the myelination patterns in adult brains might be relatively similar.
However, whether the relationship between microbiota development and neurocognitive development is limited to expansion in these brain areas is yet unconfirmed, and we still don’t know much about the exact mechanism whereby beneficial microbes possibly facilitate the development of the areas important for cognitive faculties.
What next?
A further direction for the researchers study could be observing differences in non-human primate development when the microbiota is acquired at birth compared to when it is not. It might also be interesting to tag neurotransmitters synthesised by microbes within the body and use optogenetics to monitor their effects on the development of neurons in juvenile animal brains, which would give more conclusive evidence whether the neurotransmitters produced by microbes are of large enough amounts to affect brain activity.
Nevertheless, it is still incredible to see differences in brain structure and behaviour measured so thoroughly in this study. It has forged a surprising partnership between microbiology, neurobiology and behavioural psychology research, both in terms of the topic it is investigating and the methods it has used to study this. These results certainly warrant further discussion and studies, and in the near future we may define a clear link between developmental brain physiology and the microbiome.
Further Reading
- If you are interested in learning more about the brain-gut link, this review article provides a comprehensive overview on the proposed mechanisms by which microbes interact with the brain: Grenham S, Clarke G, Cryan JF, Dinan TG (2011). Brain-gut-microbe communication in health and disease. Front Physiol 2:345-348. DOI: 10.3389/fphys.2011.00094
- And this study shows that some beneficial gut bacteria provide protective effects against Alzheimer’s disease: Nimgampalle, M., P. Kukkarasapalli, and Y. Kuna (2016). Role of Lactobacillus plantarum 46 MTCC1325 in membrane-bound transport ATPases system in Alzheimer’s disease-47 induced rat brain. BioImpacts, 2016(4): 203. DOI: 10.15171/bi.2016.27
References
- Diaz Heijtz R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016; 21(6):410-7. DOI: 10.1016/j.siny.2016.04.012
- Postma, A., Jager, G., Kessels, R. P.C., Koppeschaar, H. P.F., van Honk, J. (2004). Sex differences for selective forms of spatial memory. Brain and Cognition, 54(1): 24-34. DOI:10.1016/S0278-2626(03)00238-0.
- Neal P., Simon (2017). Periventricular/Intraventricular Hemorrhage (PVH/IVH) in the Premature Infant. Emory University School of Medicine.
- Wall R., Cryan JF., Ross Rp., Fitzgerald GF., Dinan TG., Stanton C. (2014). Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 817: 221-239. DOI: 10.1007/978-1-4939-0897-4_10
Main article
Lu J, Synowiec S, Lu L, Yu Y, Bretherick T, et al. (2018) Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLOS ONE 13(8): e0201829. DOI:10.1371/journal.pone.0201829
