Stress, Development, and the Microbiome
Stress, the sort that persists and comes from sources beyond our control, can be detrimental to our health, and even more so to the young (Luna R. et al. 2015). When outside sources of stress are present from a young age, normal development and the adult stress response is impacted in a way that makes healthy coping with stress more difficult throughout life (McEwen et al. 2011) (Eliand L. et al. 2013). Environmental stressors can not only cause developmental changes and impact overall health but can also cause changes to a person’s microbiome or the sum of microbes that coexist with an individual. Which could in-turn alter the sort of metabolites a person secretes (Verbeke K et al. 2015).

The sum of microbes that coexist with and within humans, outnumber human cells and express more genes then their human host (Martin et, al. 2018). The human microbiome is in part responsible for producing a wide range of metabolic and signaling molecules that affect one’s health and well-being, including one’s psychological state (Martinez K. et, al. 2017).
The role of the human microbiome on the human stress response is not completely understood. Scientists suspect an individual’s microbiome can be altered by an individual’s mental state in a degree dependent manner (Foster et al. 2017). The information from these interactions could travel along the gut-brain axis and are thought to happen through at least three parallel and interacting channels involving nervous, endocrine, and immune signaling mechanisms (Martin et, al. 2018). A 2020 study by Xu et al. published in Nature Scientific Reports examined young rats who were exposed to daily stress, looking for long lasting changes that occurred to their metabolism and microbiome.
Objective of Xu et al. 2020 Study
Researchers expected their test subject rats to experience strong enough physiological responses to the daily, physically induced stress that it would alter the rat’s metabolic and microbiome profiles into adulthood.
With the belief that chronic stress during a young age would alter the way their subjects’ gut-brain axis behaves, Dr. Xu and his colleagues checked their rats for long lasting changes that may have persisted from youth to adolescence and beyond. The researchers wanted to know if part of the reason development is altered is due to changes in the gut microbiome and the metabolites associated with such changes.
Evidence
By placing rats into small plastic tubes for one hour a day, researchers were able to stress the animals such that their blood corticosterone (a stress hormone that helps us adapt to our environment) rose significantly. After half of the researchers’ rats experienced 13 days of induced stress some of the subject rats were euthanized and their cecum’s were sampled. These samples provided researchers with direct access to data about the sort of microbes present in their subjects’ guts as well as the metabolites present at the time of euthanasia.
Comparing the information gathered from the stress rats to the unstressed, control rat group showed the gut microbiome compositions of the stressed rats changed— with it the sort of metabolites present in the cecum’s of the stressed rats. Dr. Xu and his team then looked for previously known microbe-metabolite pairs to try and find correlations. Specifically, correlations that would suggest the change in microbiome is altering the sort of molecules present in the cecum and vice versa. Once some pairs were chosen and a ranked list was made, statistical analysis was used to try and find which microbes may best explain the presence of certain metabolites.
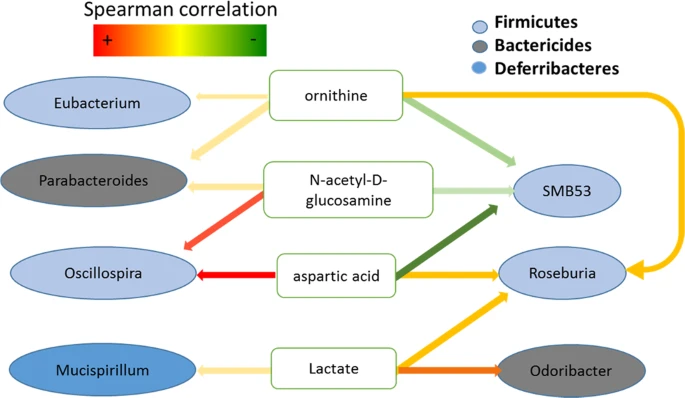
The chart labeled “Figure 6” was then produced to display some of the metabolite-microbiome pairs their data may have supported. Lactate was thought to be the most likely cause for change in cecum microbiome composition. The observed increase in lactate levels was most likely to be a result of physical restraint. These findings do not answer the researchers central question or offer much support to the claim that stress perception is altered by microbiome changes due to the nature of lactate production in mammals.
The same sort of sampling and analysis techniques were used on the group of rats euthanized three weeks after daily restraint stress treatments ended. A date chosen based on the rate of Sprague Dawley rat maturation. Interestingly, after three weeks without stress, metabolic changes persisted in the group of stressed rats even though their microbiome community structure began to return to a state comparable to their unstressed counterparts.
Dr. Xu’s work along with his colleagues’ contributions show that chronic stress induced microbiome changes may be more easily reversible then the long term metabolic changes experienced by these Sprague Dawley rats. Dr Xu’s work serves as a pilot study for future research. Paving a way for better understanding how stress, development, and the microbiome may come together to alter how one experiences stress. Dr Xu’s work highlights that improvements in animal model selection, statistical analysis, and biomarker selection may be possible and offer new ways to learn more about what role the gut-brain axis plays in our human stress response.
My Questions
- I wonder what statistical analysis will look like in the future when trying to answer questions that have as many variables as mammal development and microbiome changes?
- Will causation of human ailments be better understood as we can more closely link an individual’s microbiome to the problem a person is presenting with? Will this be possible in the short term future?
- Are taxa of microbes and the sort metabolites the right places to look to try and advance our understanding of what effects the human microbiome has on development at this point in time?
Further reading
A better understanding of what happens when humans get stressed can be gained here (Harvard Health). Similarities between the rat stress response and the human stress response can be seen here (Joëls et al. 2018). To read about some known ways the microbiome affects our mental state, click here (American Psychology Association).
References
Eiland, L, and R D Romeo. “Stress and the developing adolescent brain.” Neuroscience vol. 249 (2013): 162-71. doi: 10.1016/j.neuroscience.2012.10.048
Foster JA, Rinaman L, Cryan JF. “Stress & the gut-brain axis: Regulation by the microbiome.” Neurobiol Stress. 2017 Mar 19;7:124-136. doi: 10.1016/j.ynstr.2017.03.001.
Luna RA, Foster JA. “Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression.” Curr Opin Biotechnol. 2015 Apr;32:35-41. doi: 10.1016/j.copbio.2014.10.007.
Martin CR, Osadchiy V, Kalani A, Mayer EA. “The Brain-Gut-Microbiome Axis.” Cell Mol Gastroenterol Hepatol. 2018 Apr 12;6(2):133-148. doi: 10.1016/j.jcmgh.2018.04.003.
Martinez KB, Leone V, Chang EB. “Microbial metabolites in health and disease: Navigating the unknown in search of function.” J Biol Chem. 2017 May 26;292(21):8553-8559. doi: 10.1074/jbc.R116.752899
McEwen, Bruce S. “Effects of stress on the developing brain.” Cereb Dana Forum Brain Science (2011): 14. doi: N/A
Verbeke, K. A. et al. “Towards microbial fermentation metabolites as markers for health benefits of prebiotics.” Nutr Res Rev 28(1), 42–66 (2015) doi: 10.1017/S0954422415000037
Xu, M., Wang, C., Krolick, K.N. et al. “Difference in post-stress recovery of the gut microbiome and its altered metabolism after chronic adolescent stress in rats”. Sci Rep 10, 3950 (2020). doi: 10.1038/s41598-020-60862-1
