BACKGROUND OF ECZEMA:
The skin is our largest organ and plays an important role in our health and well being. One key role of our skin is preventing infections by acting as a physical barrier to pathogens and secreting antimicrobial enzymes in our sweat (Parham, 2015). However, whether from genetic or environmental factors, sometimes this defense system goes wrong.
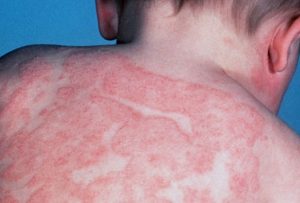
Eczema is an umbrella term for a group of diseases that result in the inflammation of the skin (atopic dermatitis or AD). According to the American Academy of Dermatology, AD is a common skin disease in children, affecting up to 20% (American Academy of Dermatology, 2018). Most kids grow out of the disease, but somewhere between 10 and 30% do not (Eichenfield et al., 2014).
AD is characterized by red, dry and chronic inflammation of the skin which leads to or worsens the skin barrier impairment. A dysfunctional skin and anti’microbial barrier provides a feasible route for microorganisms to come into contact with our immune system (Thomas et al., 2017). Although, AD was initially thought to be an intrinsic immune system abnormality, investigation into the microbiome is increasingly drawing attention to a relationship present between a microbial imbalance and AD (Thomas et al., 2017).
THE CENTRAL QUESTION:
Enshi Zhang and collaborates did a study in 2011 that addressed the relationship between pathological conditions and skin microbiota in AD patients. They asked, how does the skin fungal microbiota compare between mild, moderate, or sever AD patients and healthy individuals?
EVIDENCE:
They found that Malassezia was the major microorganism of the skin microbiota in both patients and healthy individuals. Malassezia is a yeast that constitutes most of the fungal microbiota of healthy human skin, however Malassezia may cause or worsen AD symptoms. The authors found a higher proportion of Malassezia in healthy individuals compared with AD patients (around 80% compared to 70%). Yeasts other than Malassezia were more diverse in the patients with AD compared to the healthy individuals.
Patients with mild or moderate symptoms tended to have similar fungal communities whereas patients with severe symptoms were separated into different groupings. Similarly, the healthy individuals were separated into another group. These finding suggests that the fungal microbiota of the skin varies within patients of the same disease according to the disease severity.
The authors discuss that differences in microbiota might be due to the physical condition of the skin, such as pH. Healthy skin is weakly acidic, whereas patients with AD have neutral pH skin. This less harsh pH facilitates the invasion of external microorganisms, including Staphylococcus aureus. S. aureus is believed to be a trigger for AD due to its increased colonization with severity. Staphylococcus epidermidis, on the other hand, decreases with increase severity of AD. This is suggested by the weighted presence of Staphylococcus aureus and Staphylococcus epidermidis.
Additionally the authors discuss how the amount of antimicrobial enzymes may also affect fungal microbiota. Antimicrobial enzymes are produced in healthy skin to inhibit pathogen growth. However, patients with AD are unable to efficiently produce these enzymes, allowing pathogens to colonize the skin that would typically be exterminated. This may explain in part why the fungal microbiota of these patients is different from that of healthy individuals.
MY QUESTIONS:
This raises the question that if the antimicrobial enzymatic activity in sweat is restored will symptoms of eczema be reduced? Dermcidin, an antimicrobial protein, is continuously expressed in sweat glands and secreted in sweat. Rieg and collaborators found that AD patients have a reduced output of dermcidin which contributes to their compromised innate skin defense and altered skin colonization. Sweating in healthy patients results in a reduction of viable bacterial cells on the skin’s surface; perhaps a cream that contains dermcidin can be used to imitate the release of the protein by sweating (Reig, 2005). I would suggest a study that observes if this cream treatment would reduce the severity of eczema symptoms by targeting the microbial community present. In my proposed study there would be two treatment groups (dermcidin containing cream and plain cream) that would be applied to healthy individuals and AD patients with mild, moderate and severe eczema. The overall goal of this experiment is to observe a shift in the relative abundance of the microbial communities of AD patients towards that of healthy individuals.
Another question that comes to mind is how does the application of steroids, a typical treatment for eczema, affect the skin microbiota? Zhang et al. mention that their AD patients were treated with medium to strong steroid ointments but avoided daily skin care in their study of skin fungal characterization. I believe further testing on AD patients regarding the effect of steroids of varying strengths would be a good study to follow up their experiment.
FURTHER READING:
- If you would like more background on eczema I suggest this short YouTube video by ‘Osmosis’ explaining the causes, symptoms, treatment and diagnosis of eczema (AD).
- If you are interested in reading more on how the microbial colonization of AD skin is influenced by reduced production of antimicrobial peptides in sweat perhaps give “The role of microorganisms in atopic dermatitis” paper by Baker (2006) a try. For more information on the role of Staphylococcus aureus and other bacteria and fungi within the skin microbiome perhaps try reading “The microbiome and atopic eczema” by Thomas and Fernández’Peñas (2017).
REFERENCES:
- American Academy of Dermatology, Retrieved 2018
- Eichenfield LF, Tom WL, Chamlin SL, et al. (2014), Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol; 70(2):338—351
- Parham, P. (2015). The immune system. Garland Science: New York. ISBN: (Paperback) 978-08153443667
- Rieg, S., Steffen, H., Seeber, S., Humeny, A., Kalbacher, H., Dietz, K., … & Schittek, B. (2005). Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. The Journal of Immunology, 174(12), 8003-8010.
- Thomas, C. L. and Fernández’Peñas, P. (2017), The microbiome and atopic eczema: More than skin deep. Australasian Journal of Dermatology, 58: 18-24. doi:10.1111/ajd.12435
- Zhang, E. , Tanaka, T. , Tajima, M. , Tsuboi, R. , Nishikawa, A. and Sugita, T. (2011), Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiology and Immunology, 55: 625-632. doi:10.1111/j.1348-0421.2011.00364.x
